SEVIA-USA WELCOMES YIHI!!
SEVIA-USA is proud to welcome it’s latest member… Yihi!
Dimitris and I have been working on this for a long time and it has finally happened. Yihi is now a member of SEVIA-USA, helping us in our fight against the unjust and overreaching FDA regulations.
Welcome Yihi and THANK YOU!
A BATTERY MOOCH POST: Avoid using the Efest SODA charger at its 1A setting
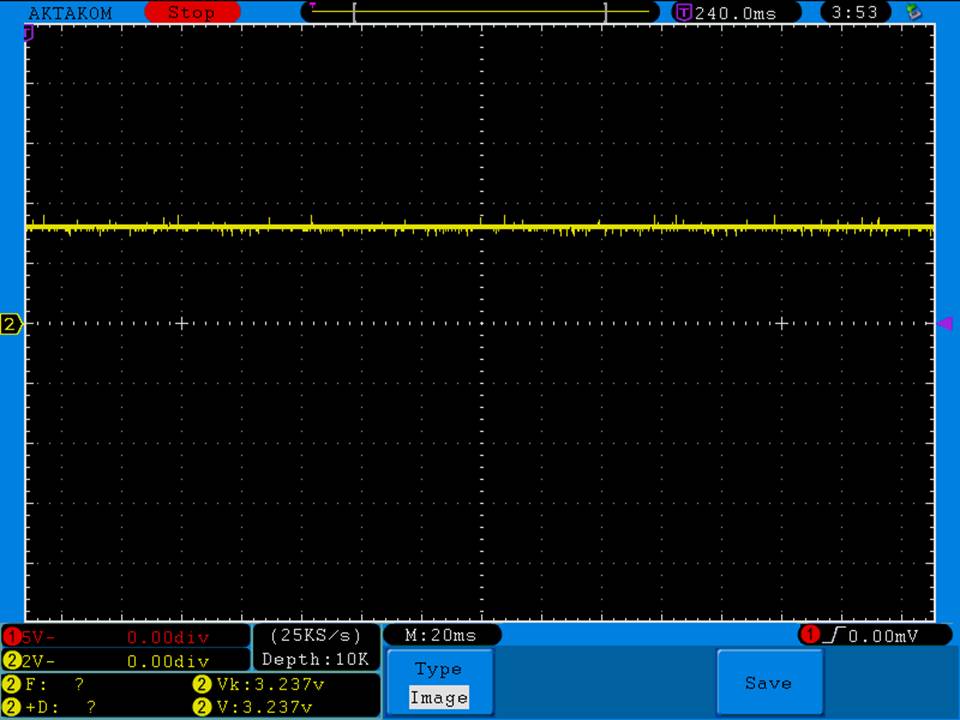
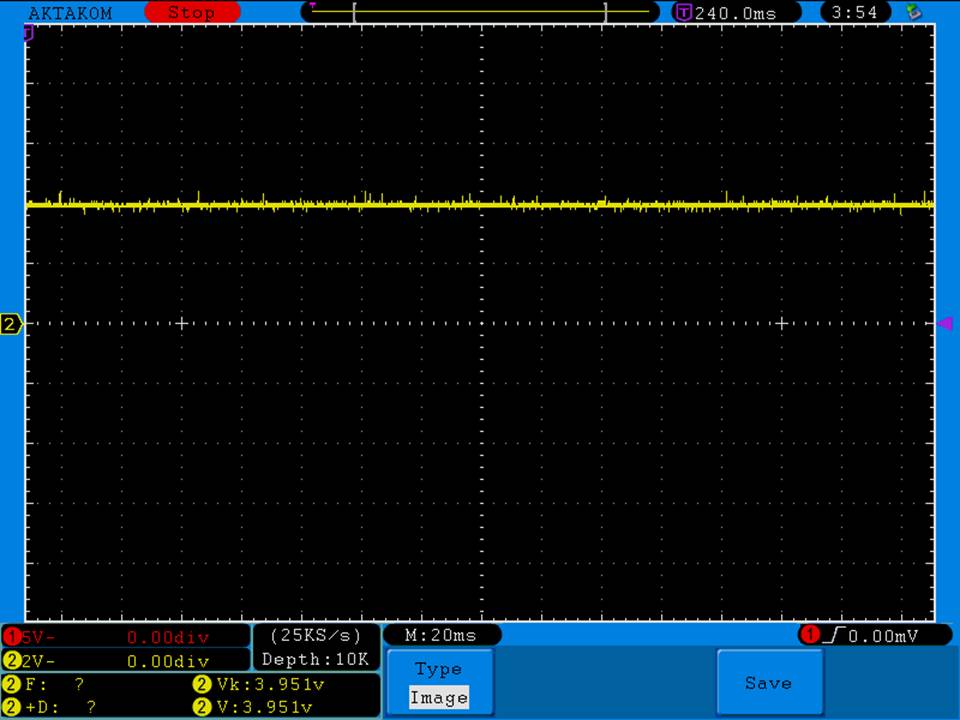
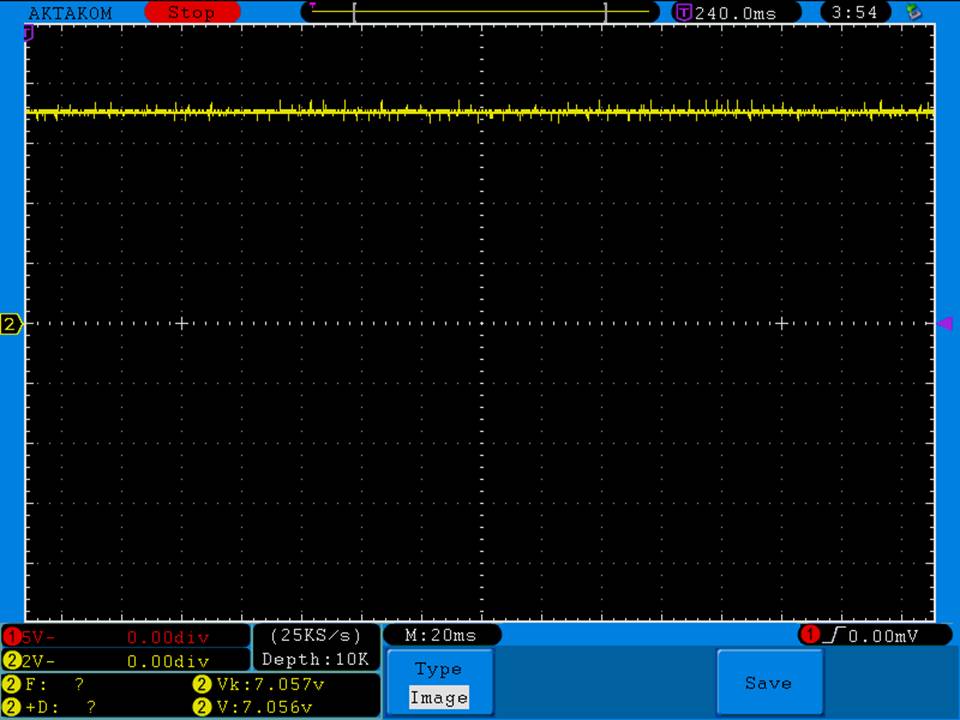
I have seen a couple of mentions of overheating and failure with Efest SODA chargers so I decided to check into it.
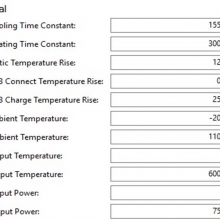
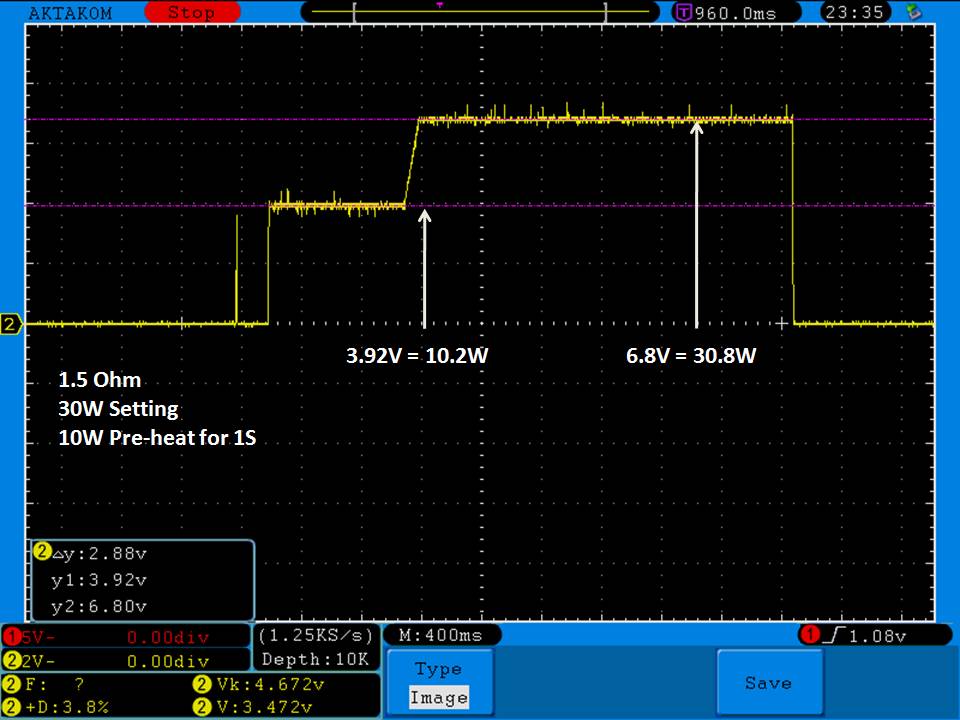
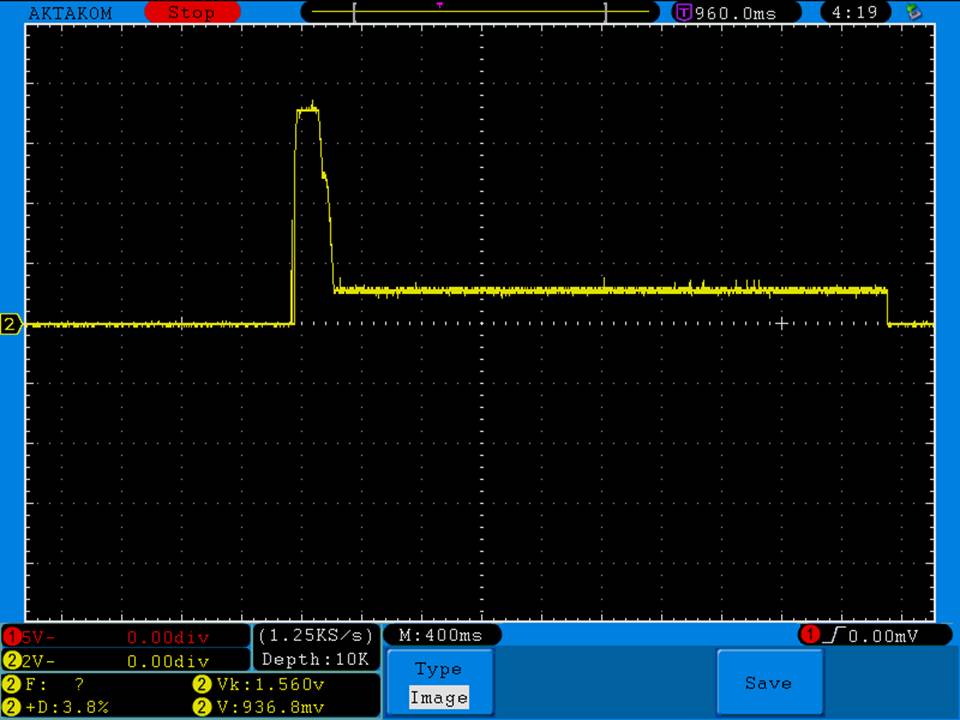
I measured the external and internal temperatures of two SODA chargers at their 0.5A and 1A settings, charging two batteries at once. They were only about 3°C apart for any temperature reading so I averaged the two readings.
Ambient temperature = 27°C
Temperatures at 1A:
Top external = 71°C
Bottom external = 87°C
Internal = 108°C
Batteries = 53°C
Those charger and battery temperatures are too high!
The bottom was hot enough to be painful to touch and the internal temperature exceeded the 105°C rated max of the capacitors inside (see the photos). Those capacitors are quite temperature sensitive and shouldn’t be run at anywhere near their max rating to ensure a long life. That high of a temperature is also bad for every other component in the charger.
In addition, the batteries were being heated up above the 45°C (approximately) point where aging of the batteries starts accelerating. The measured temperature of 53°C is what I would call “very warm”.
In my opinion, this charger runs too hot at the 1A setting and could fail prematurely because of that. It will also heat your batteries up enough to possibly shorten their overall life some. I advise running the Efest SODA charger only at its 0.5A setting.
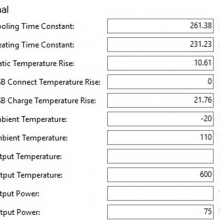
Charger temperatures at 0.5A:
Top external = 49°C
Bottom external = 55°C
Internal = 71°C
Batteries = 38°C
Running it at the 0.5A setting does not guarantee that nothing can go wrong!
I still recommend always charging on a non-flammable surface and staying nearby until charging is done. Then remove the batteries from the charger and store them in a non-conductive case or sleeve. Do not store your batteries in the charger!
Never put any charger in a LiPo battery charging bag!
Those bags are made to only hold the battery pack. Putting the charger and batteries in the bag traps all the heat from the charger inside and the temperatures can rise high enough to cause failure of the charger.
Images: https://imgur.com/a/DG90C
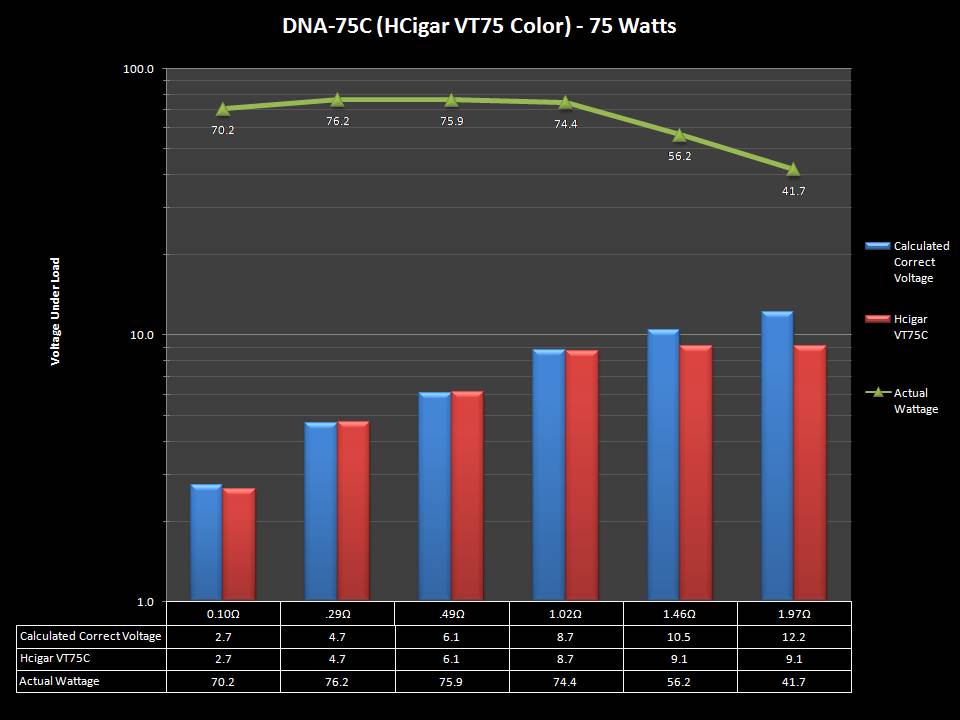
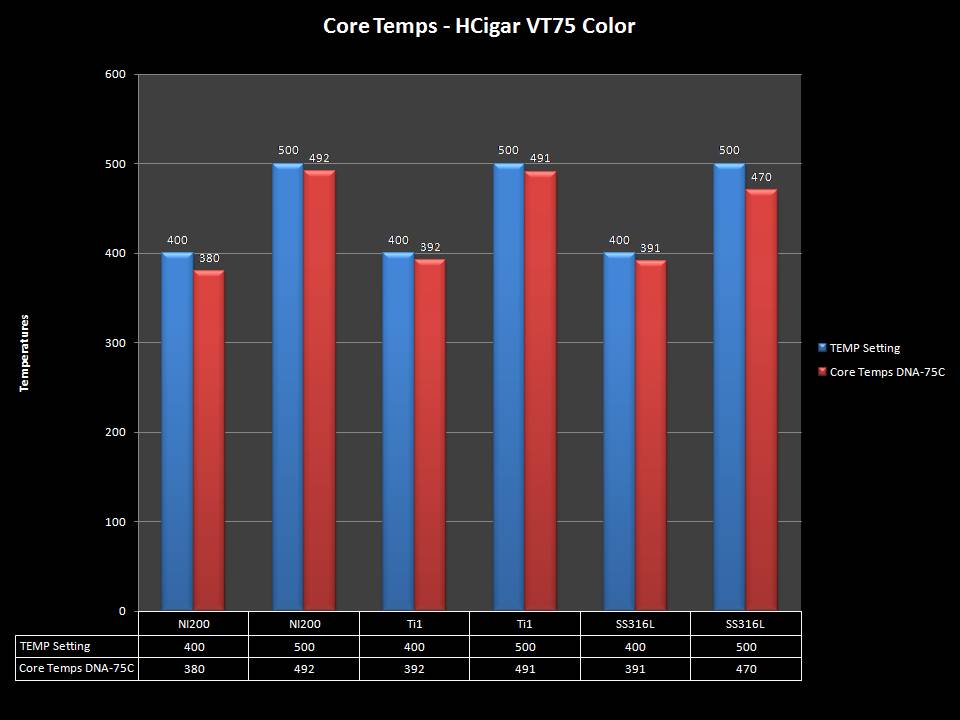
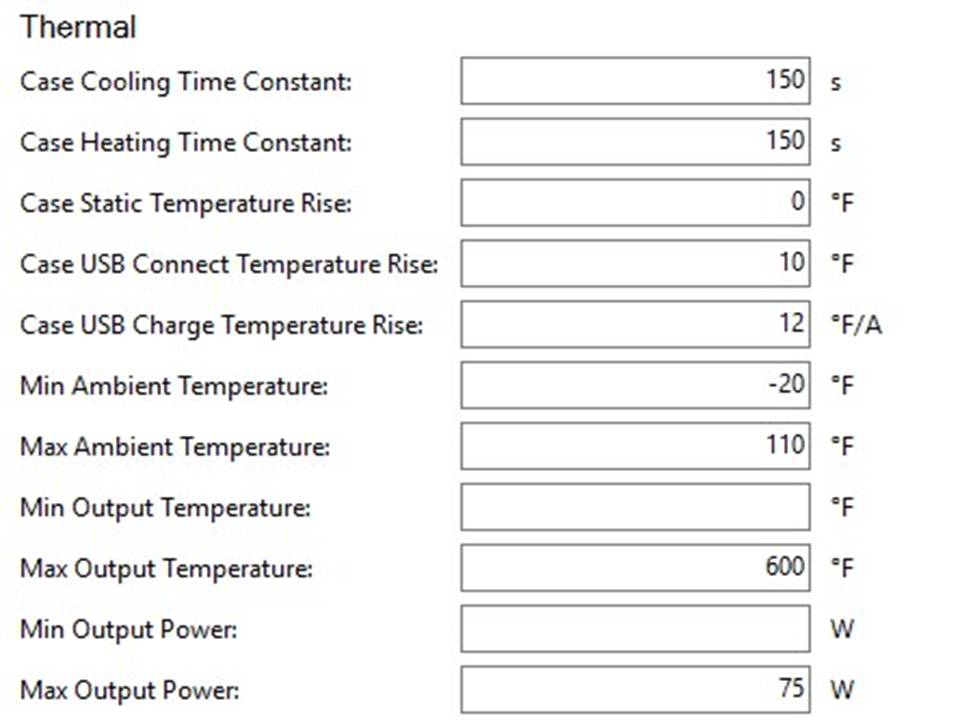
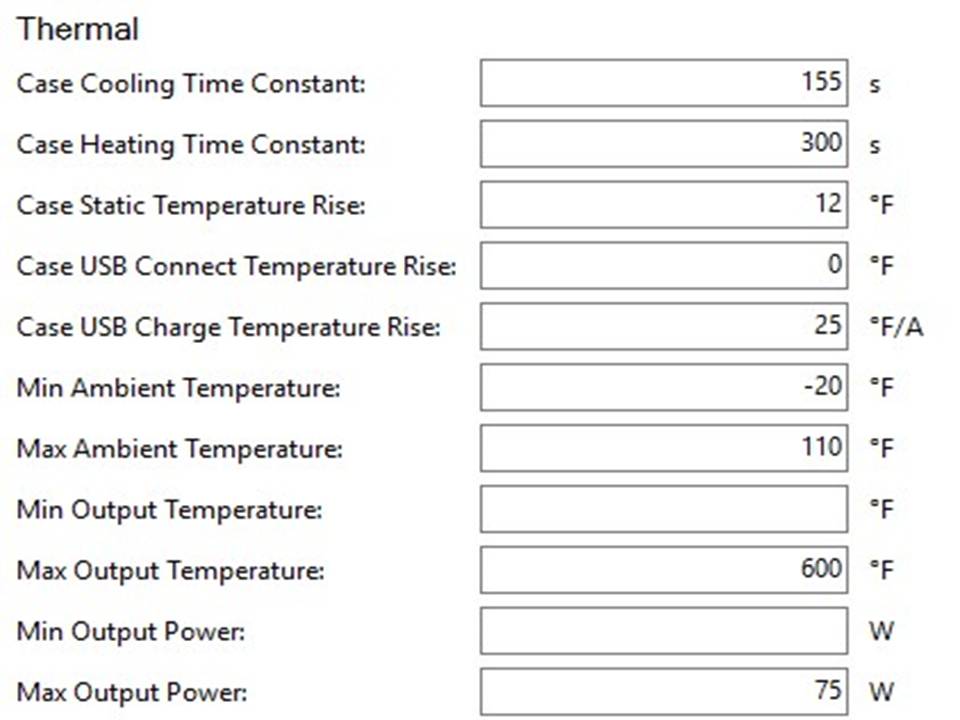
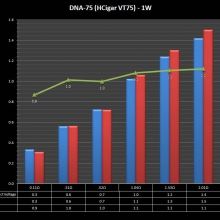
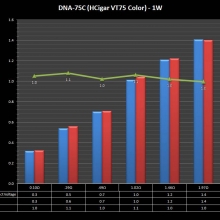
THE HCIGAR VT75 COLOR
A PBusardo Review – The HCigar VT75 Color
In this video we take a look at the new HCigar VT75 Color.
The Links:
HCigar
Element Vape
OBS
The Video:
*NOTE: Any use of these videos in part or in their entirety without Phil Busardo’s expressed written consent is strictly prohibited.
The Photos:
NEW FROM REGULATOR WATCH – Dirty Tactics | How Climate Change Science Polluted Vaping
Here’s the latest from Brent Stafford at Regulator Watch:
Did climate change politics poison science? According to Dr. Roy Spencer, the lead scientist on NASA’s best instrument for monitoring arctic sea ice and global ocean temperatures, the answer is yes.
Join RegWatch for an in-depth analysis of the break down in scientific integrity and how the breach polluted anti-vaping research at its core.
Learn the principles that drive anti-vaping hysteria and how they directly link to the battle tactics mastered by the forces fighting climate change—only on RegWatch by RegulatorWatch.com.
RegulatorWatch.com – June 7, 2017.
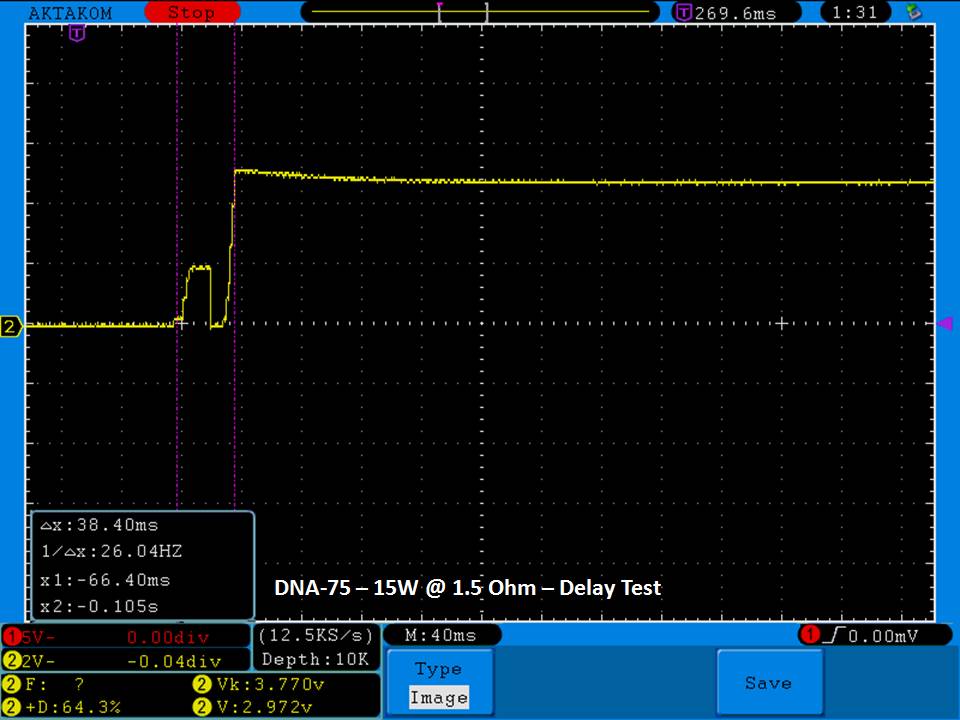
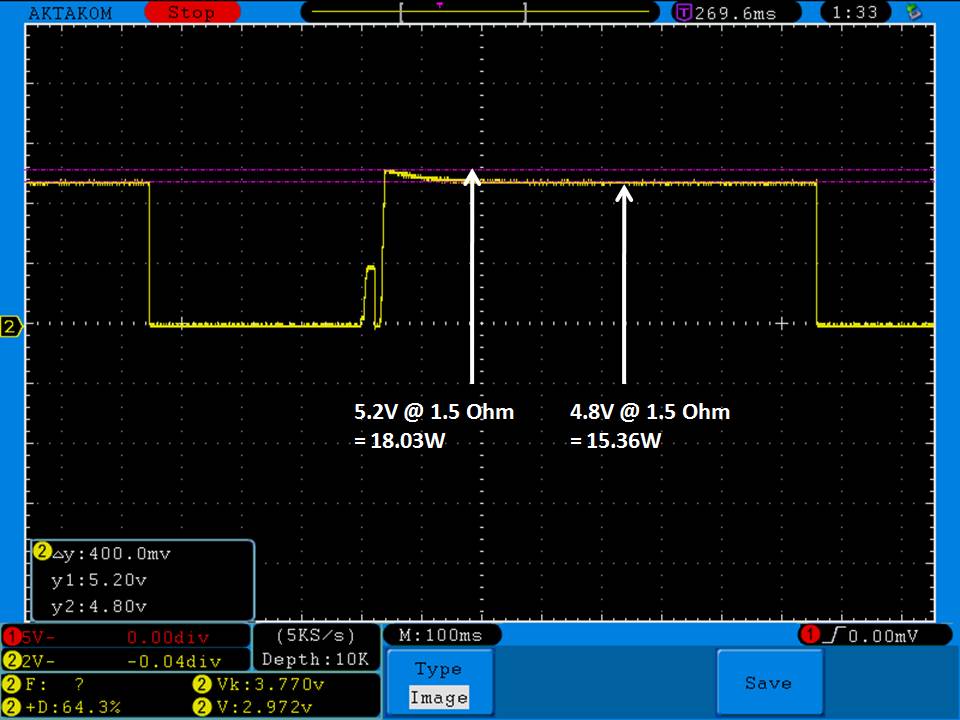
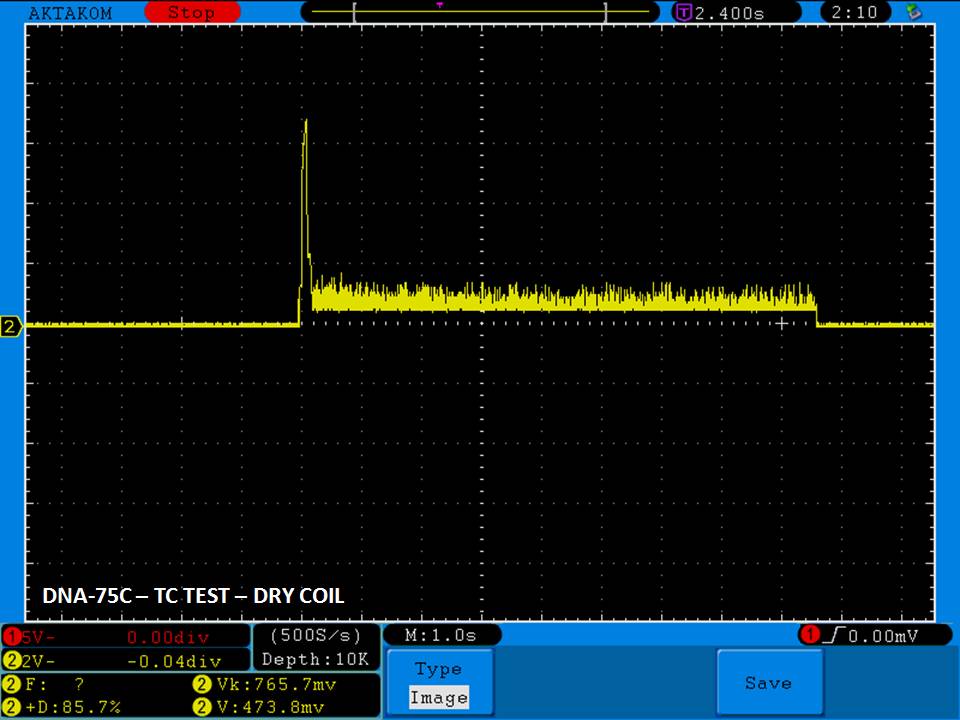
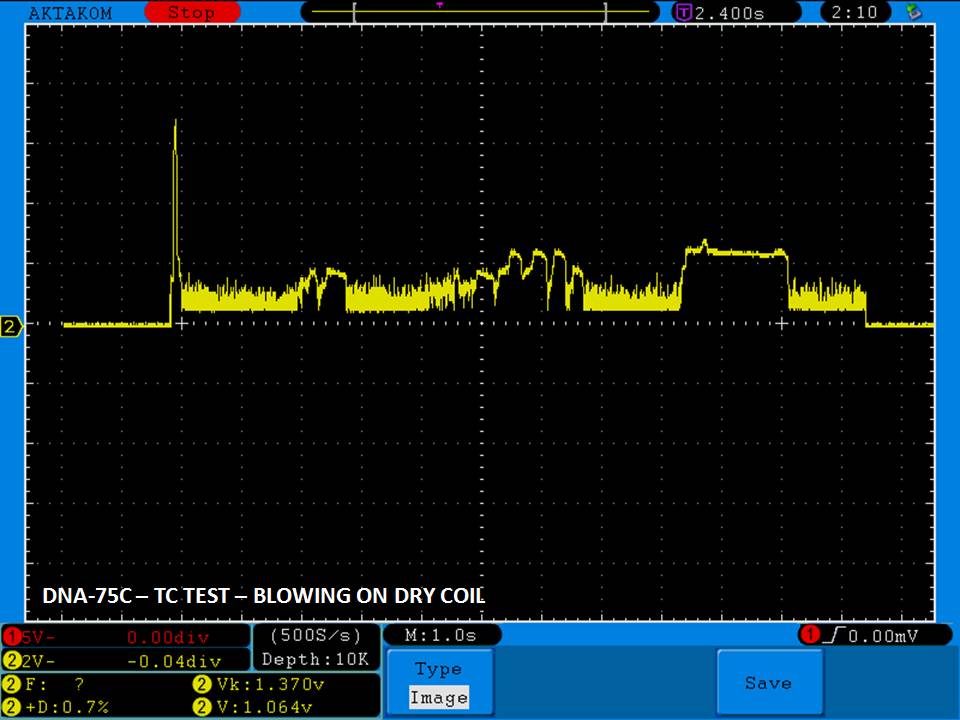
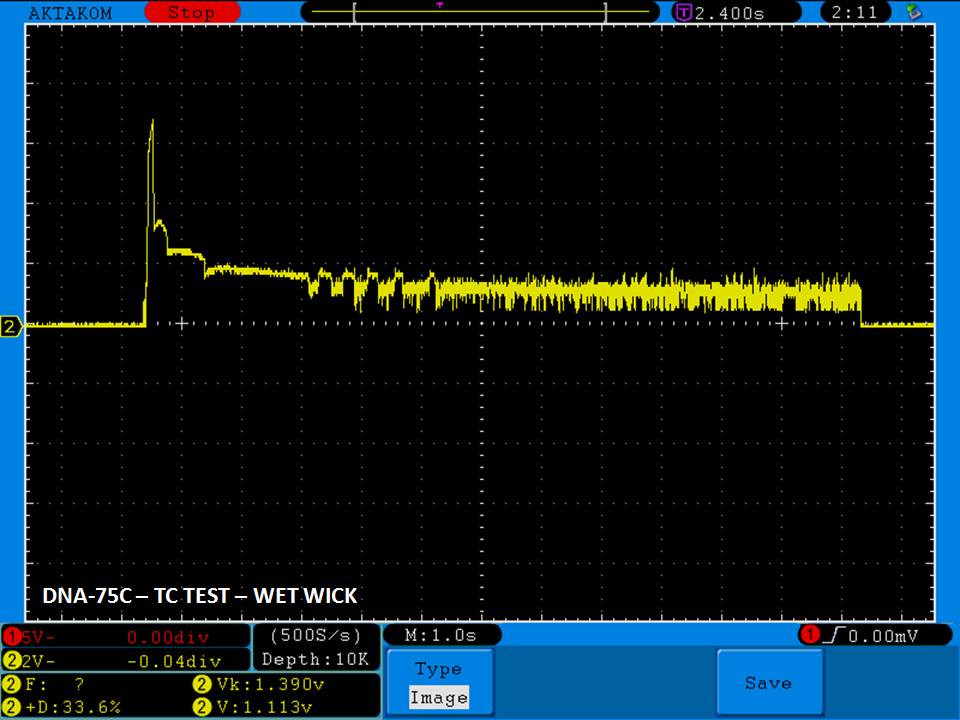
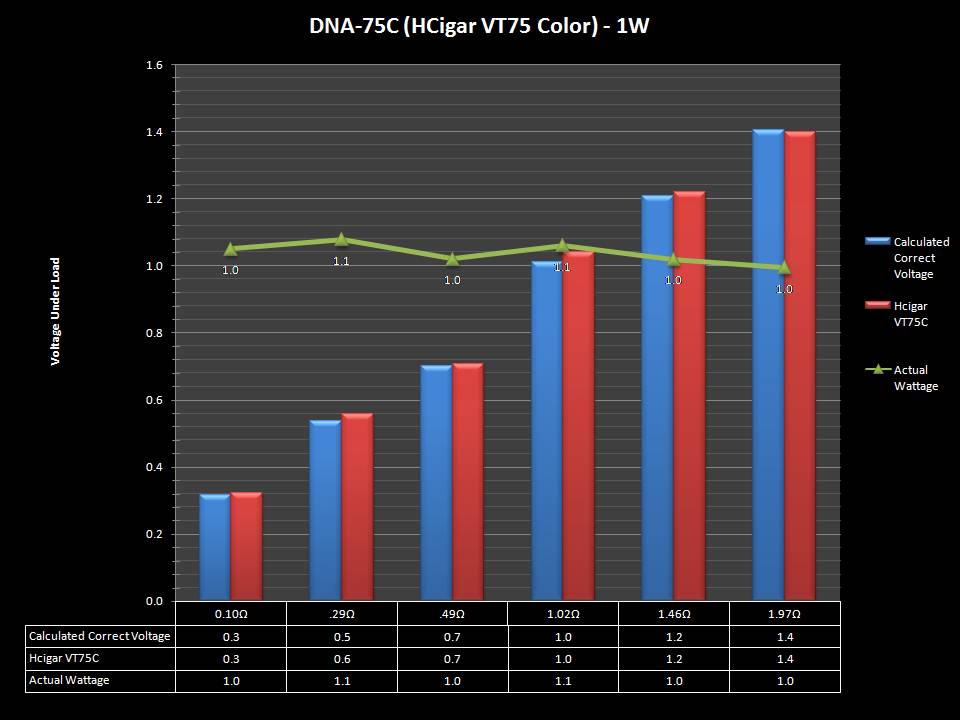
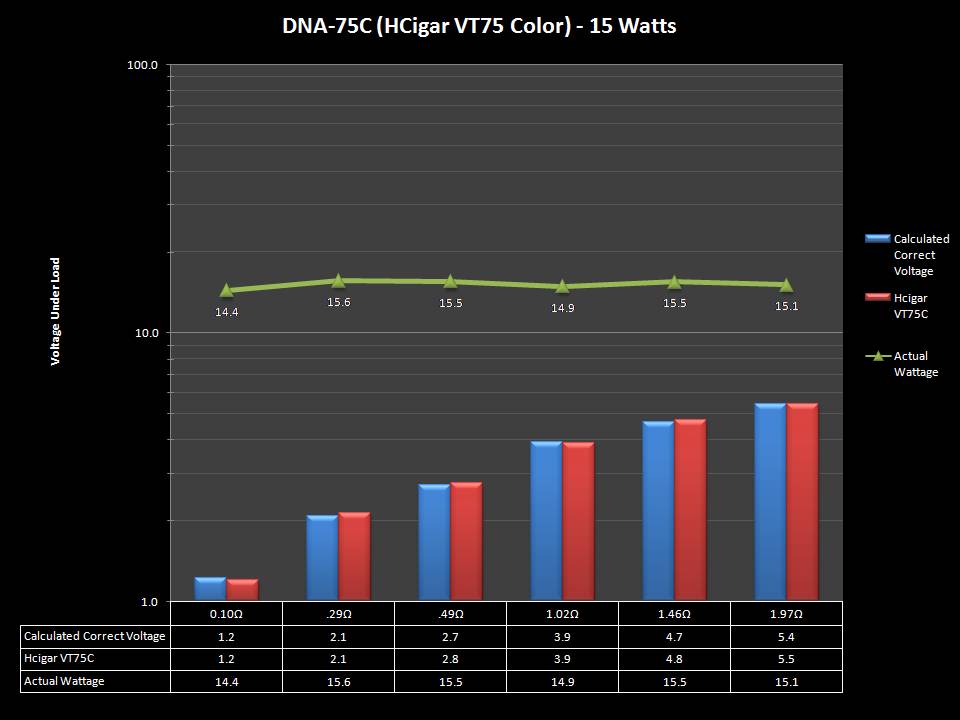
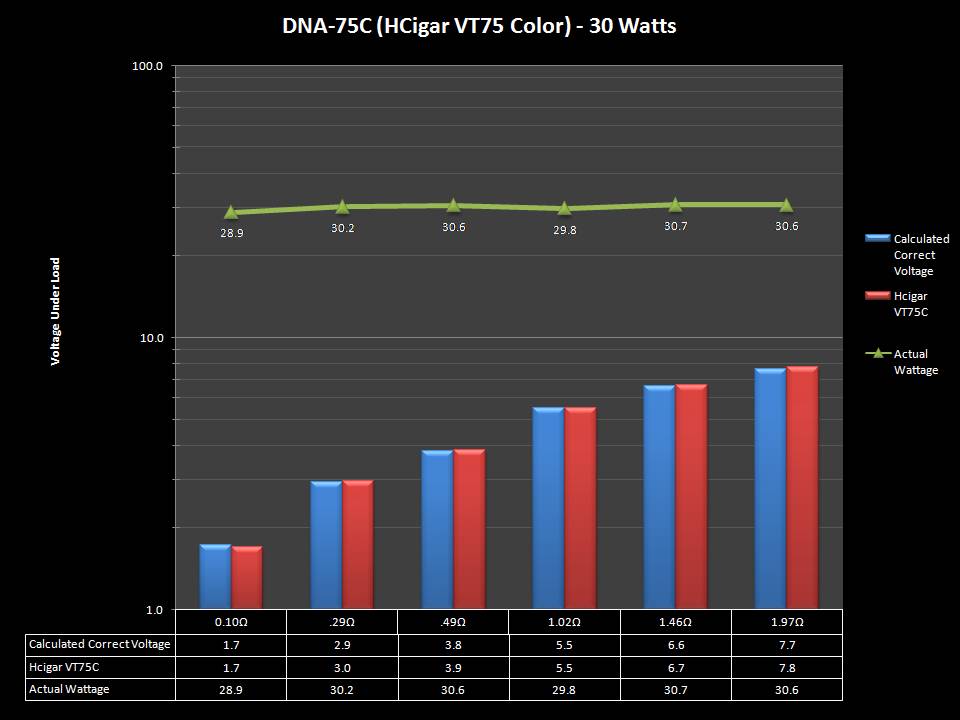
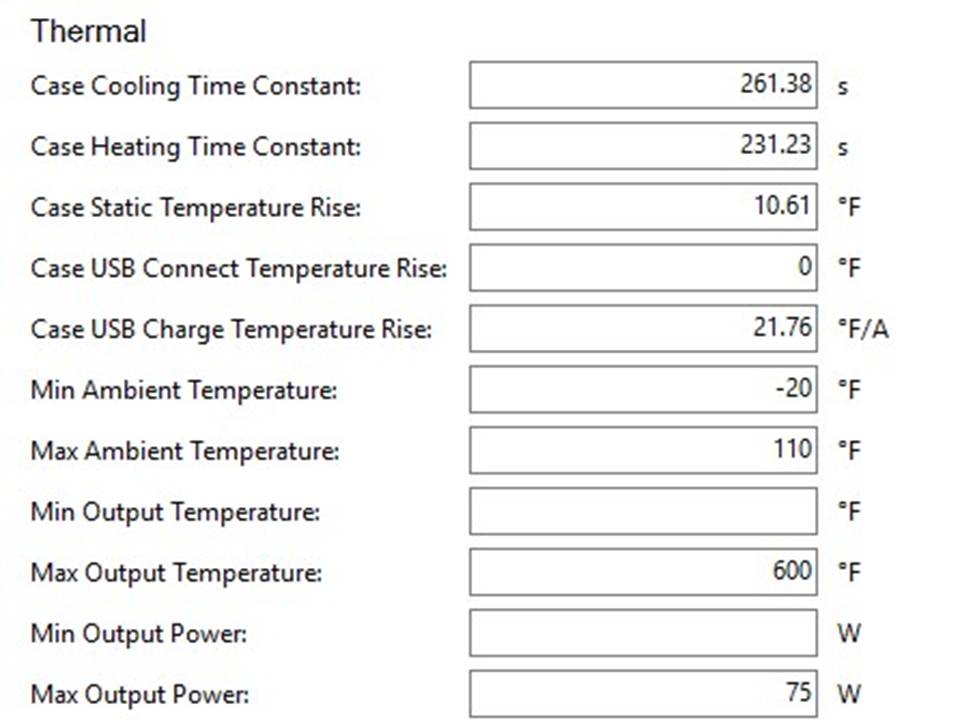
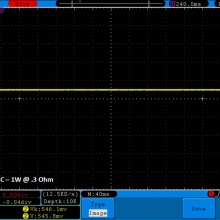
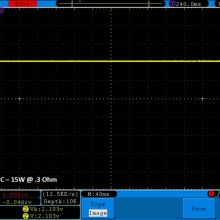
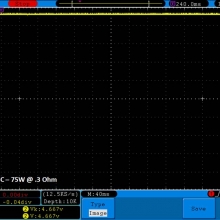
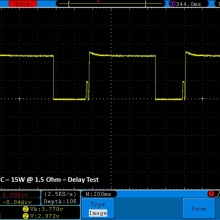
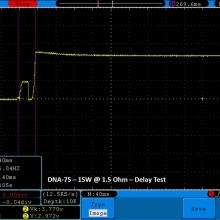
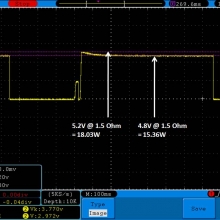
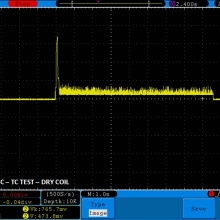
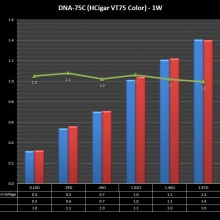
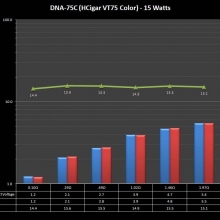
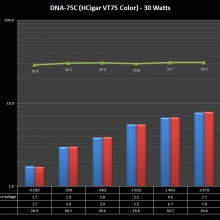
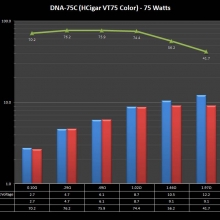
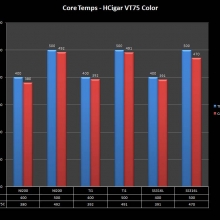
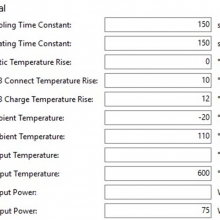
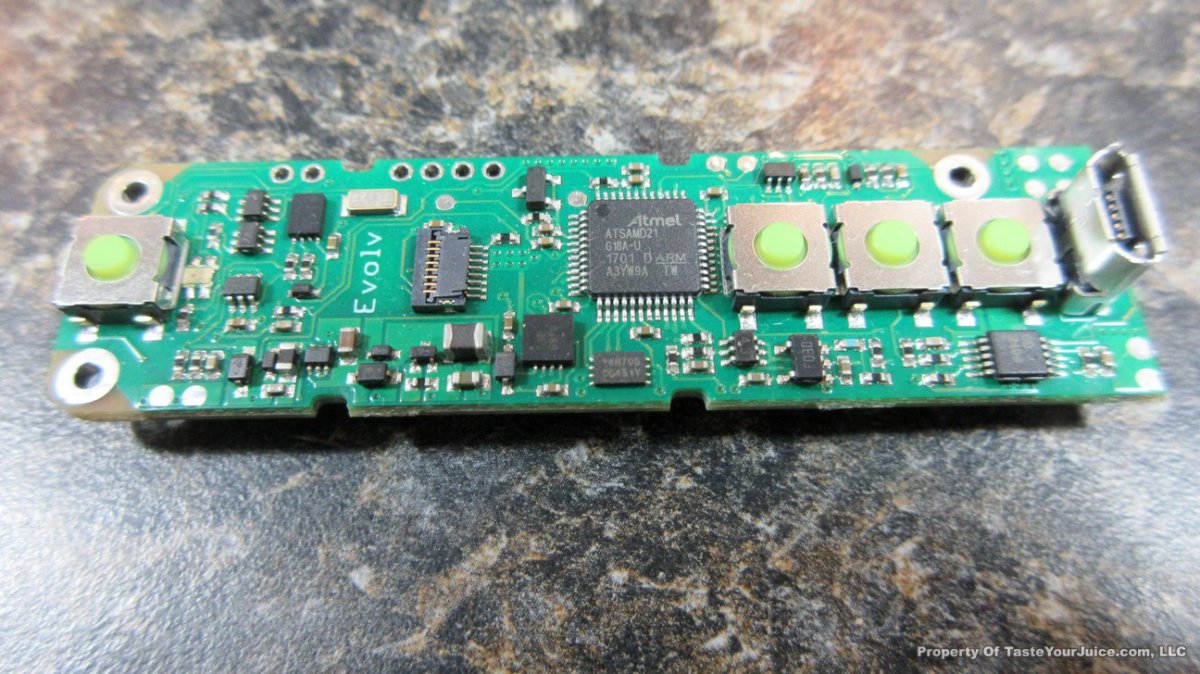
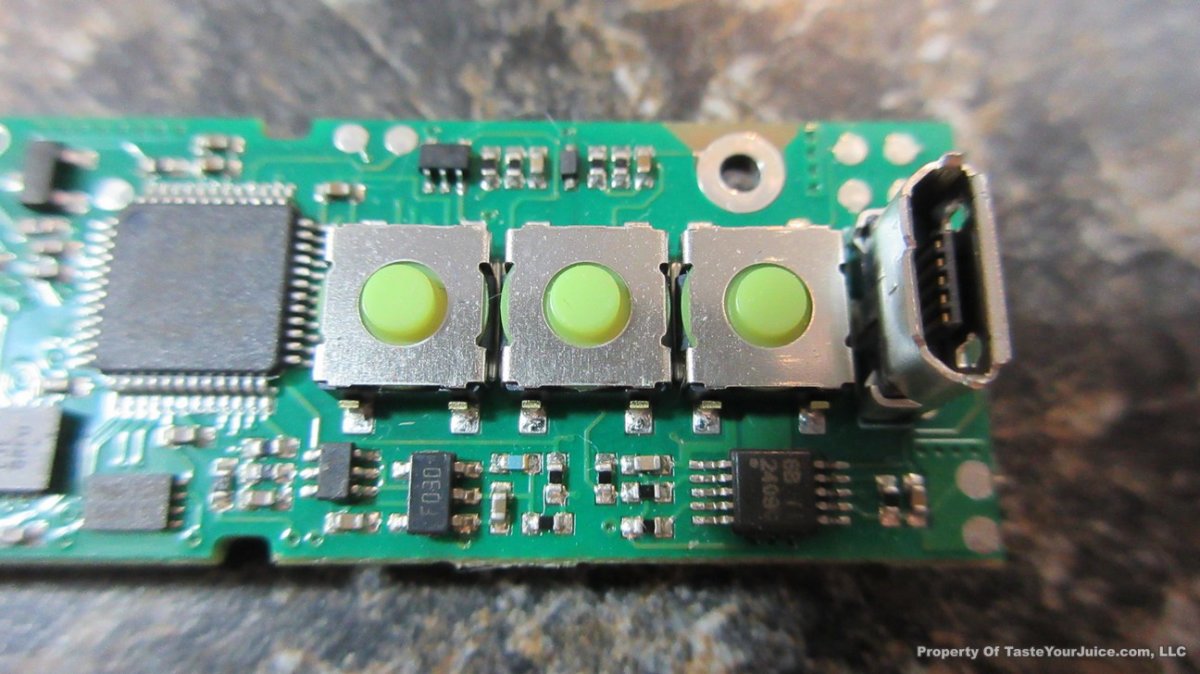
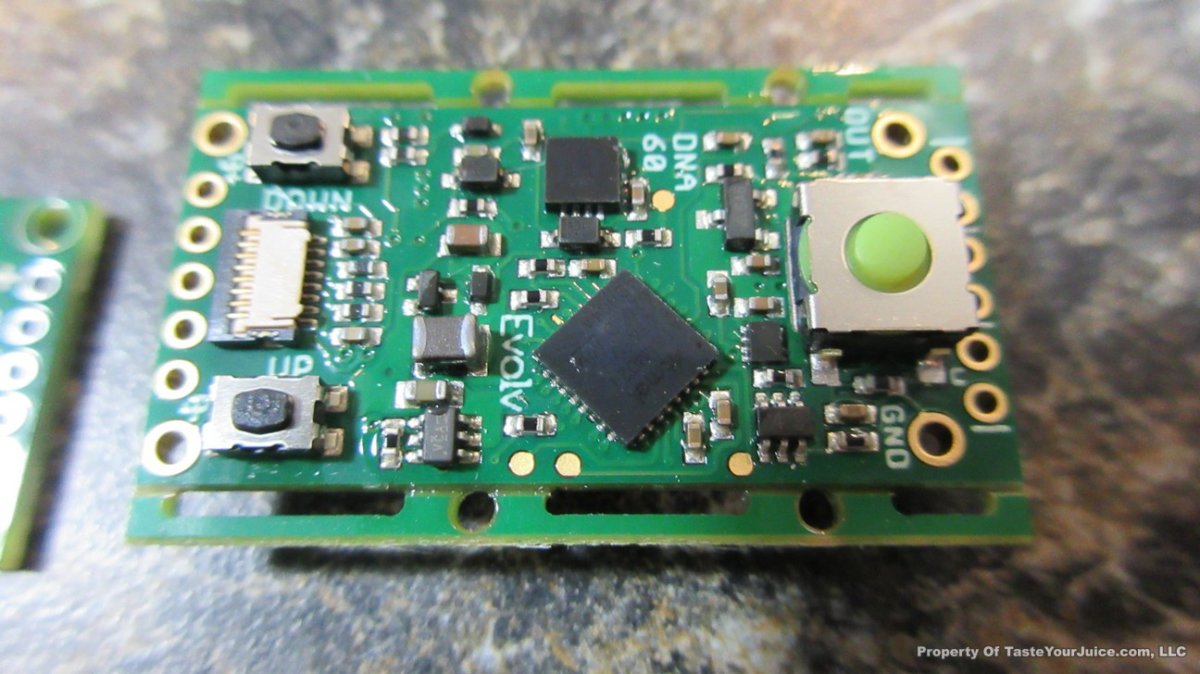
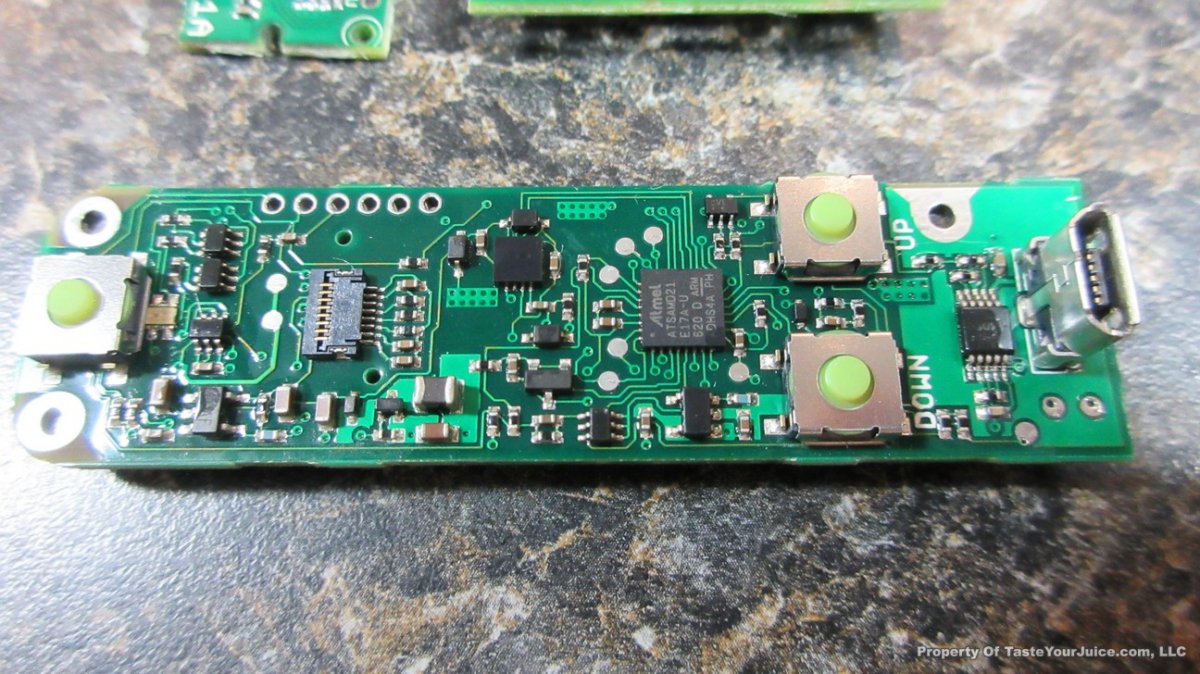
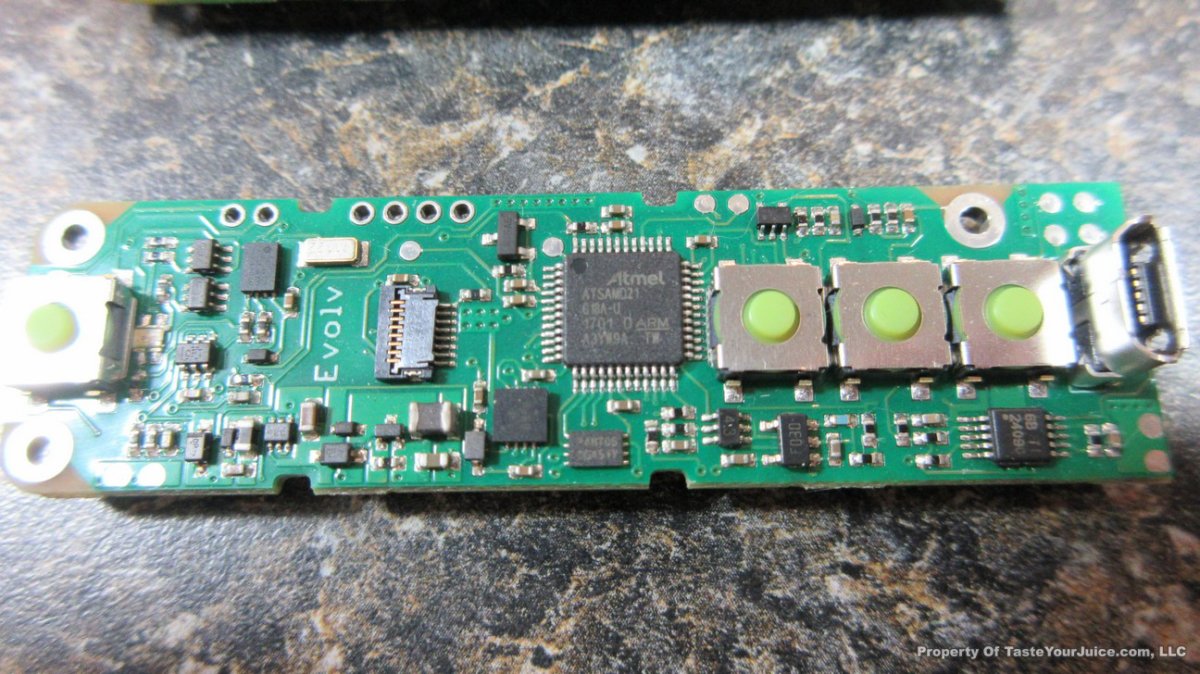
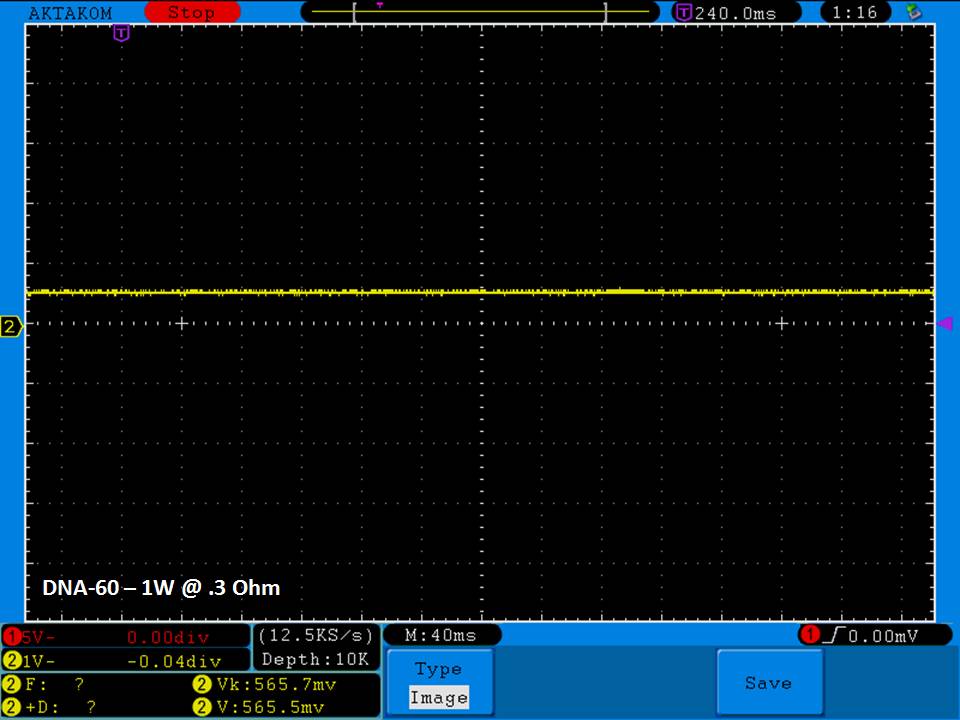
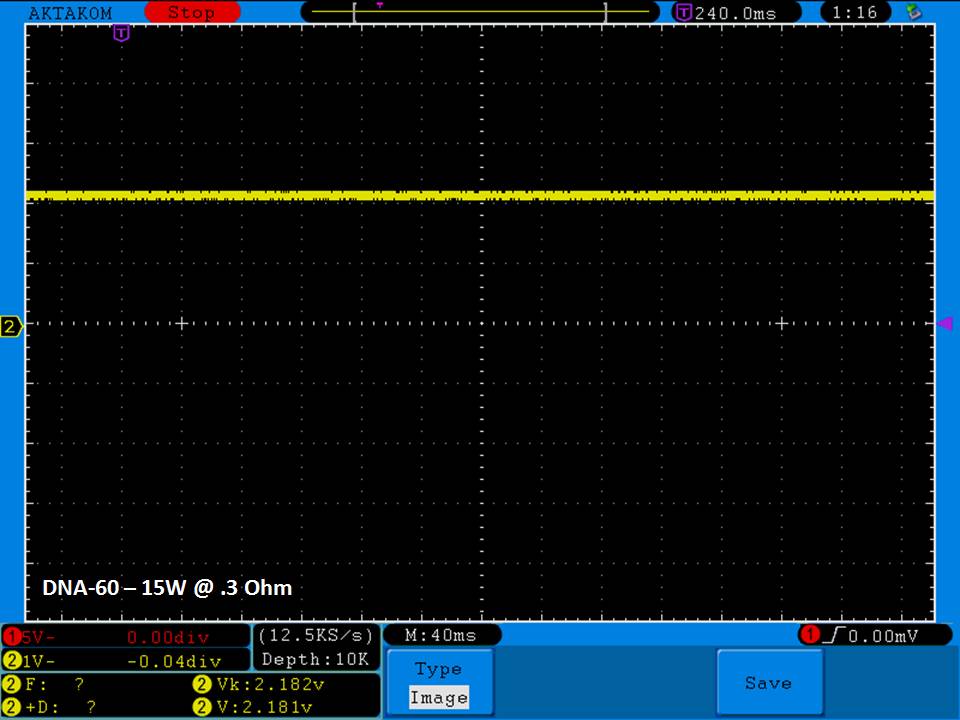
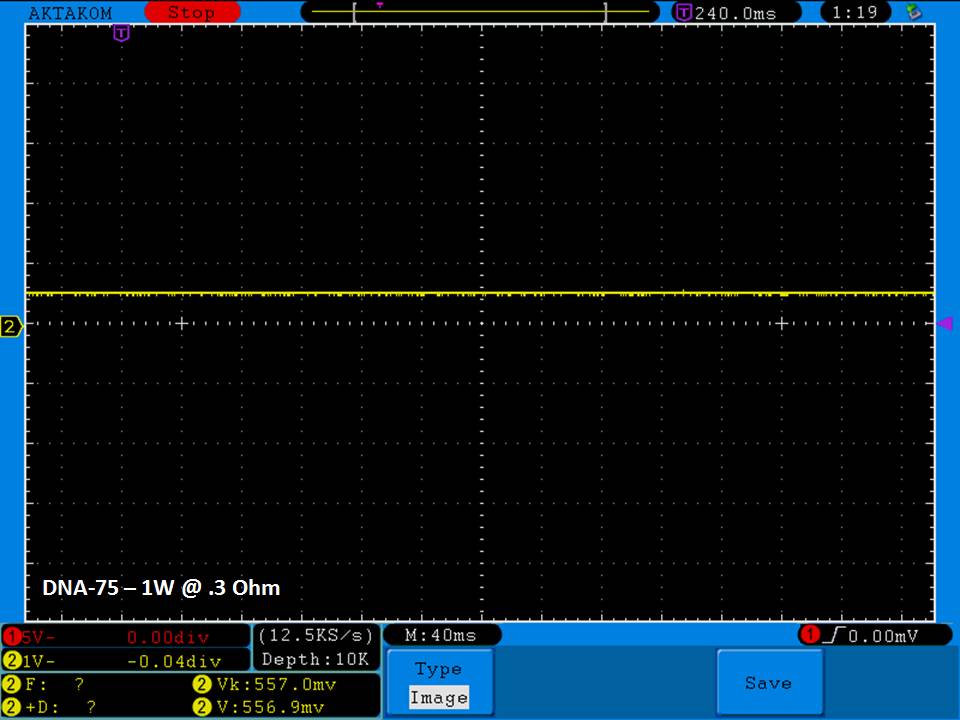
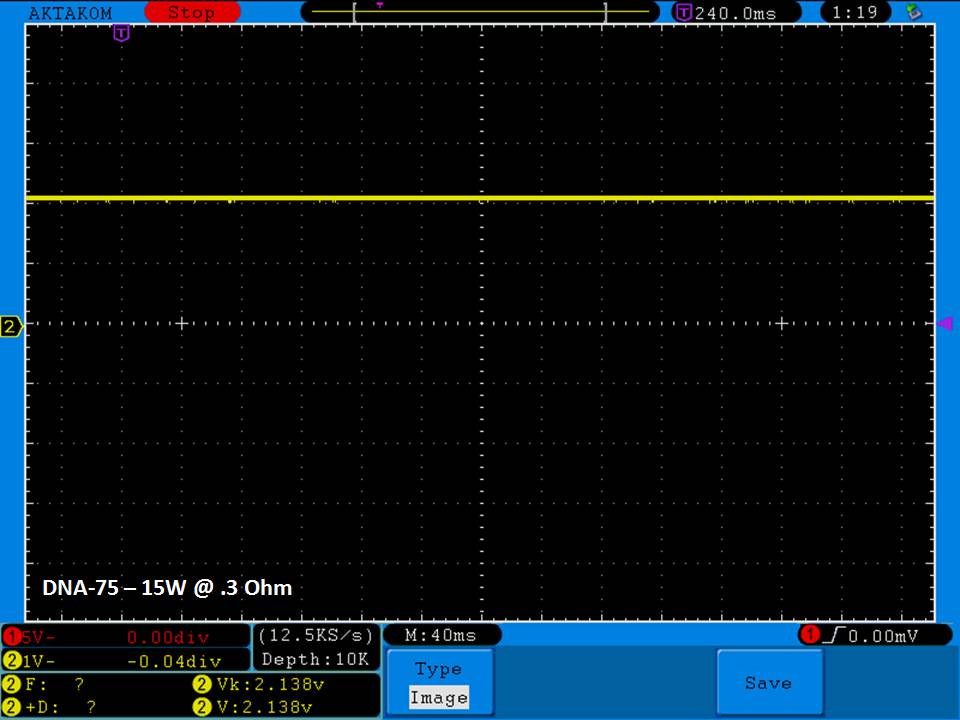
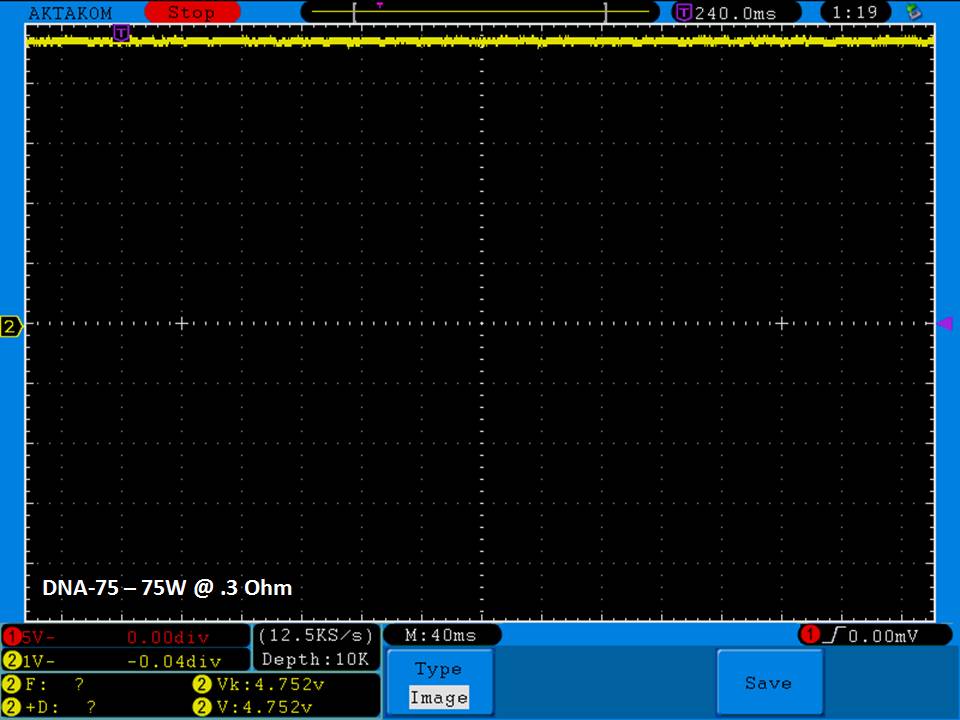
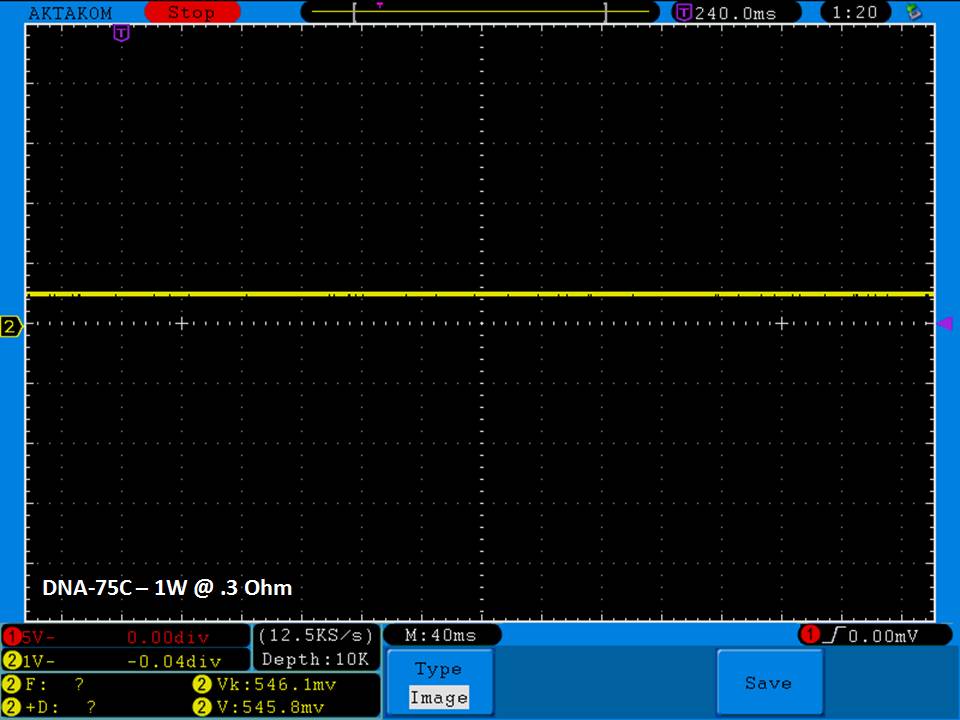
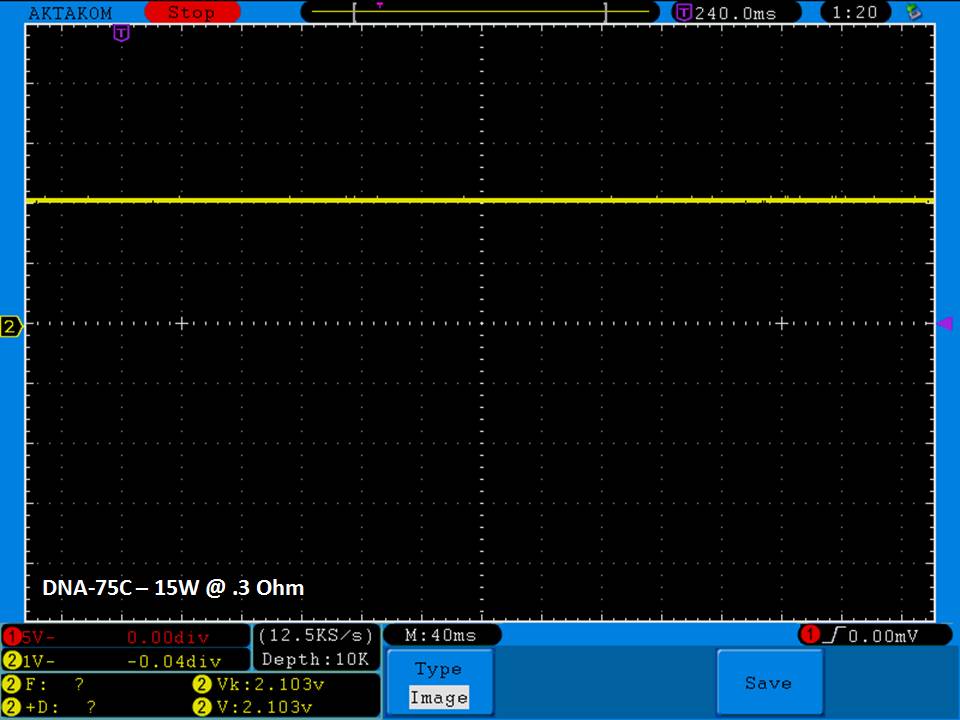
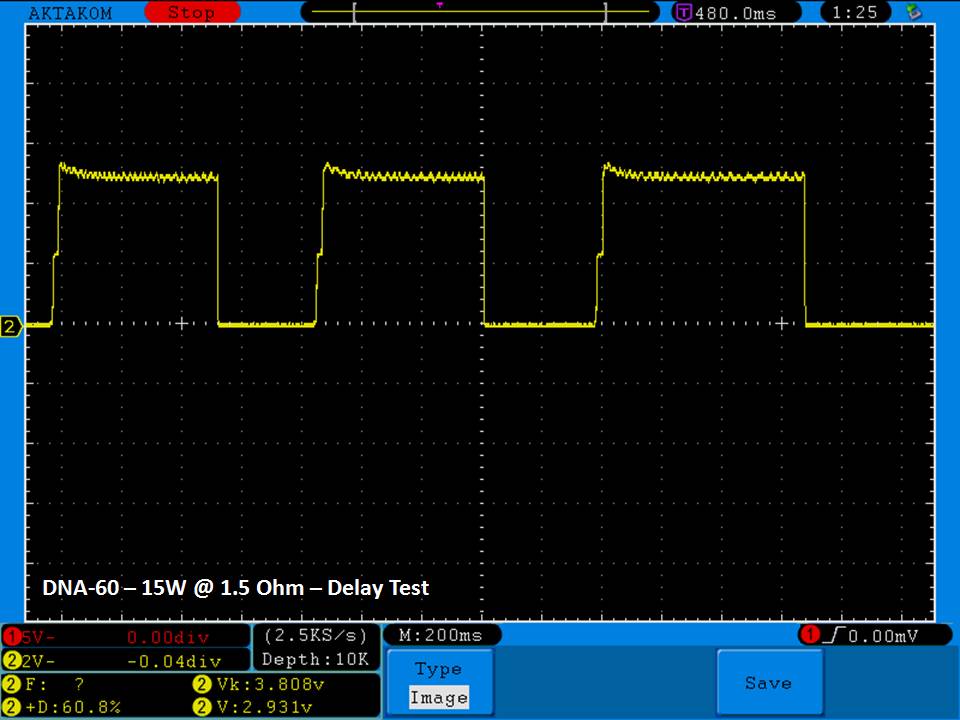
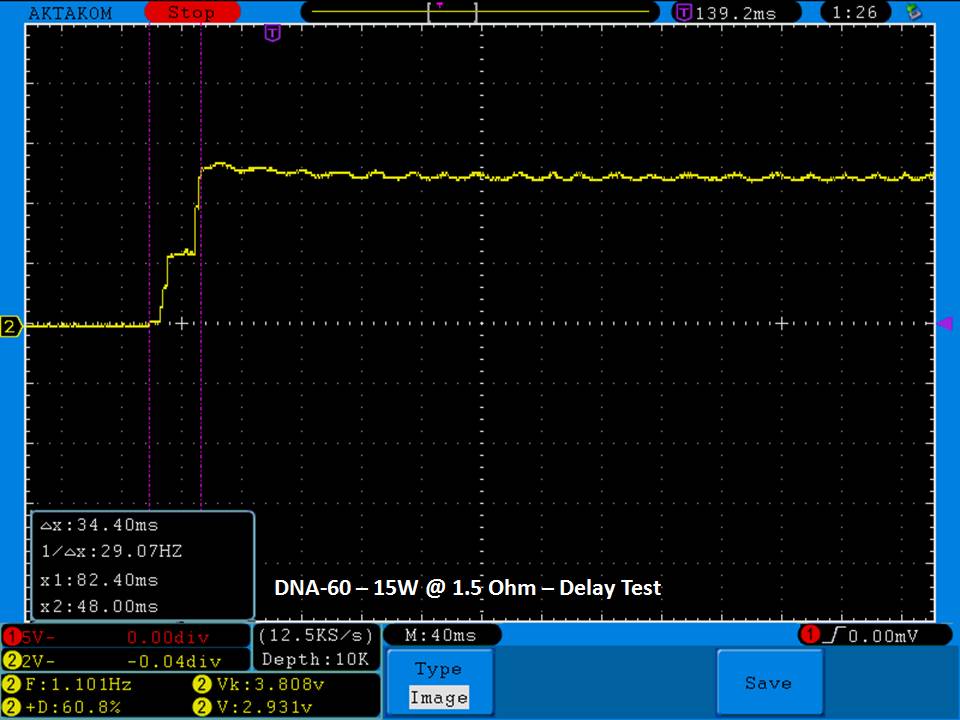
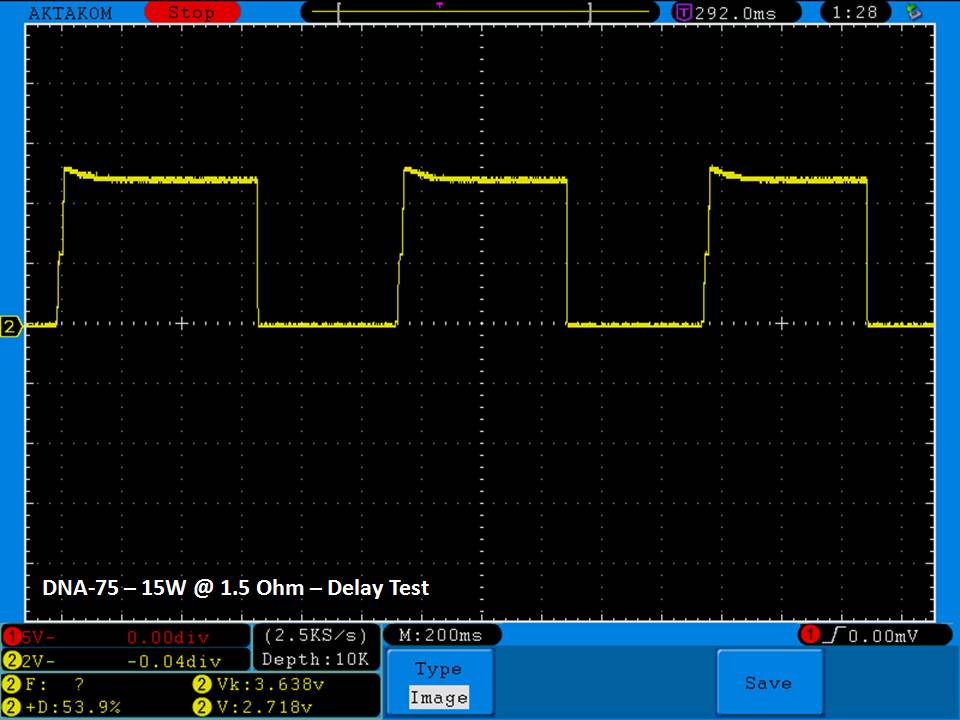
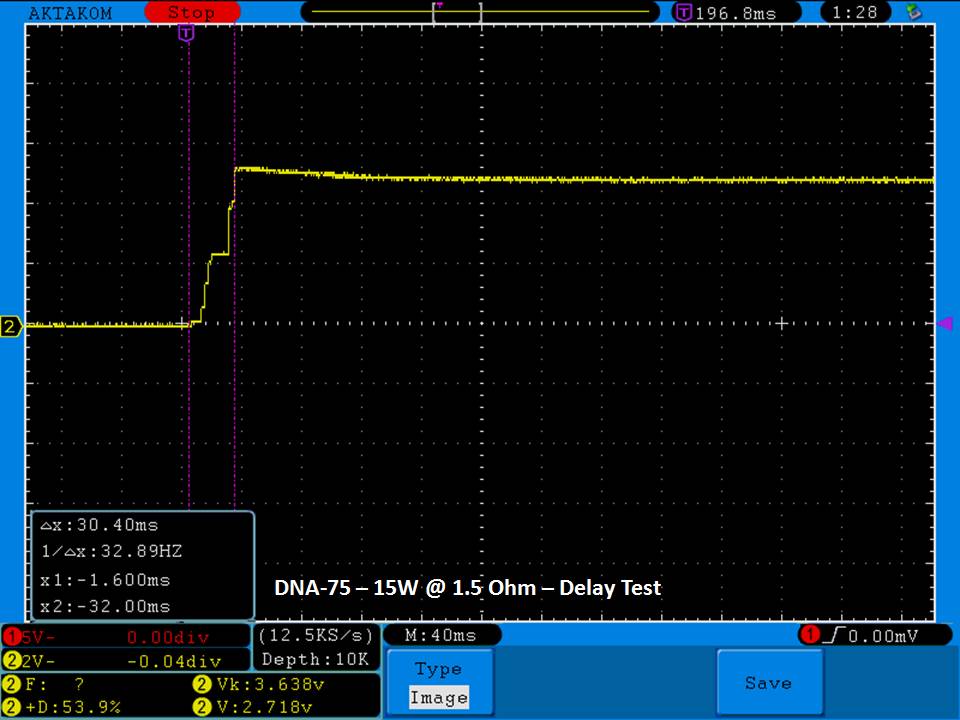
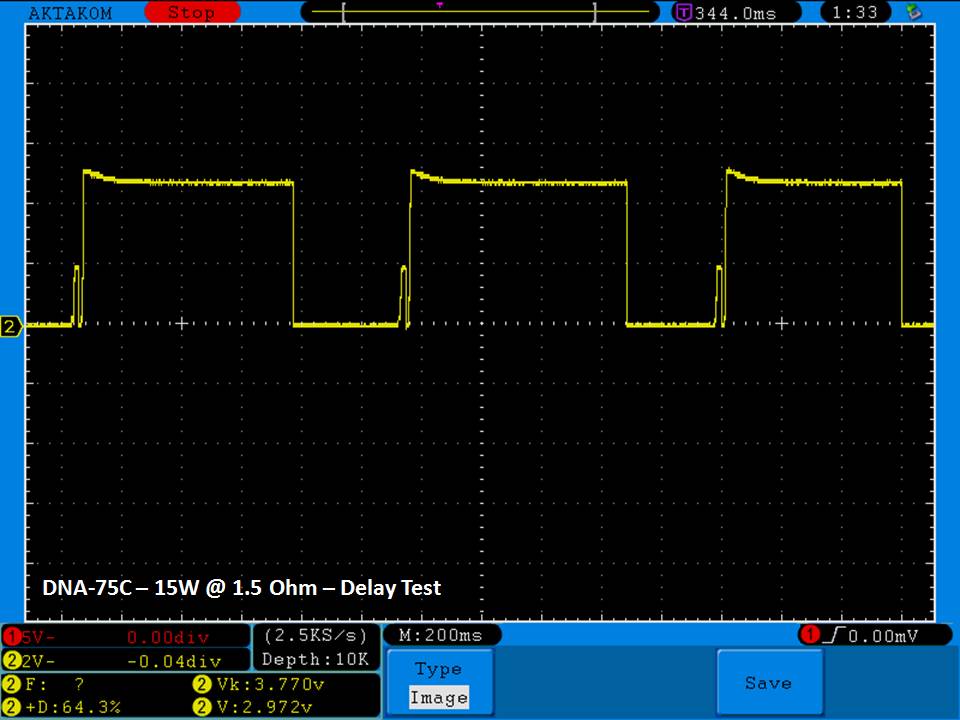
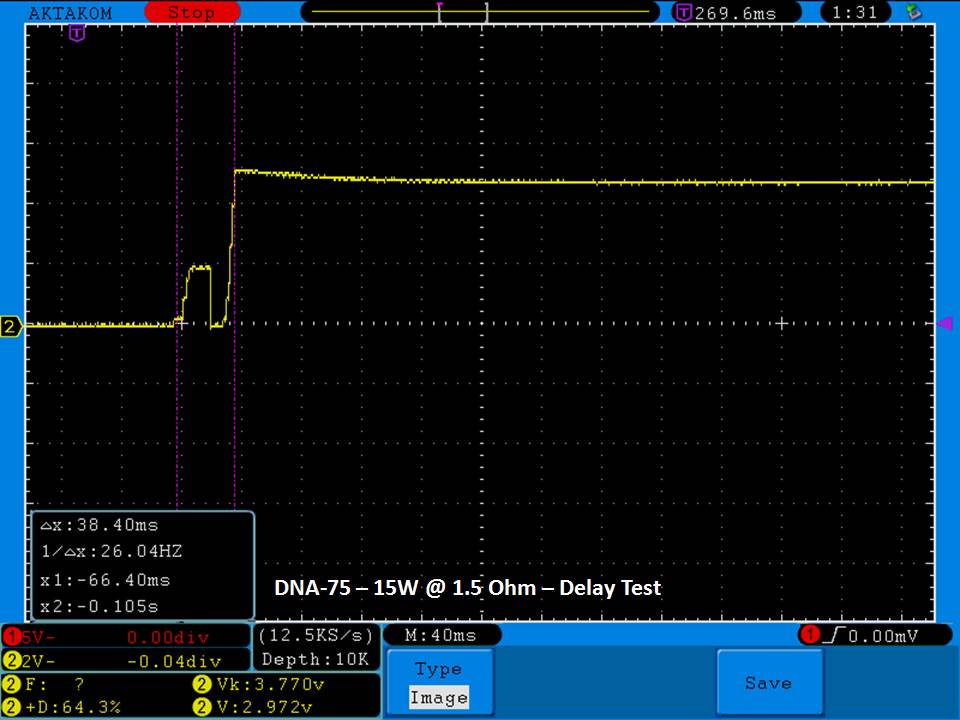
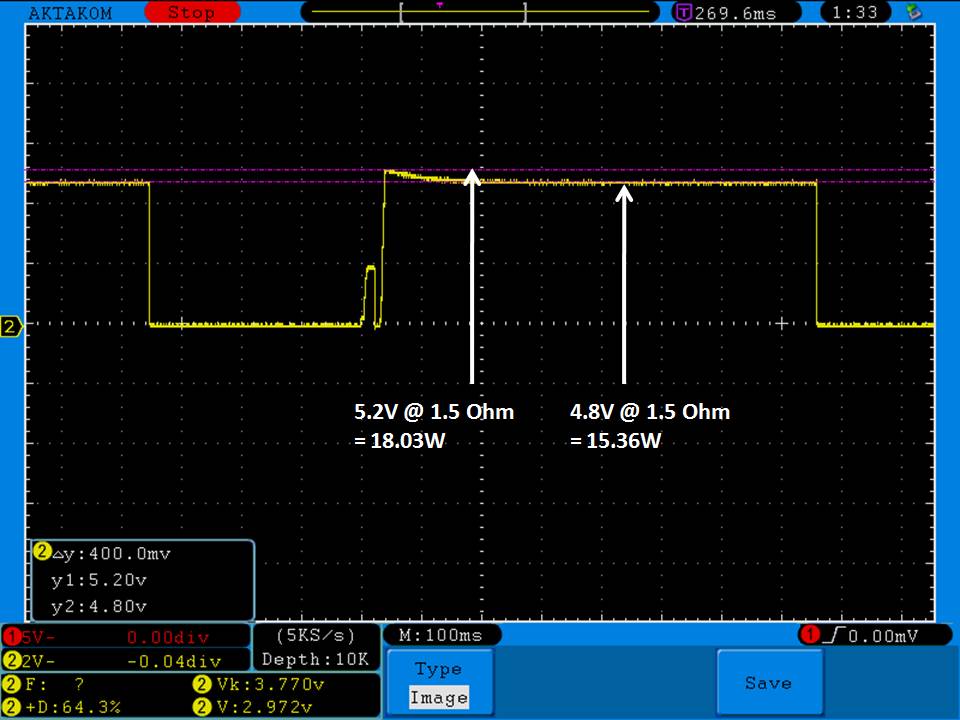
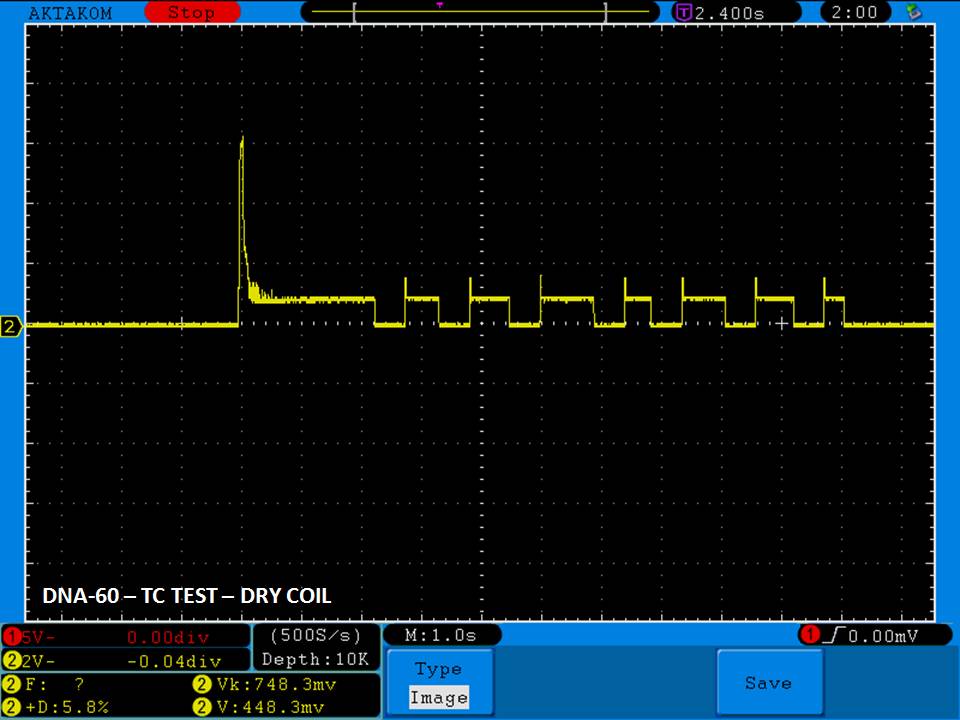
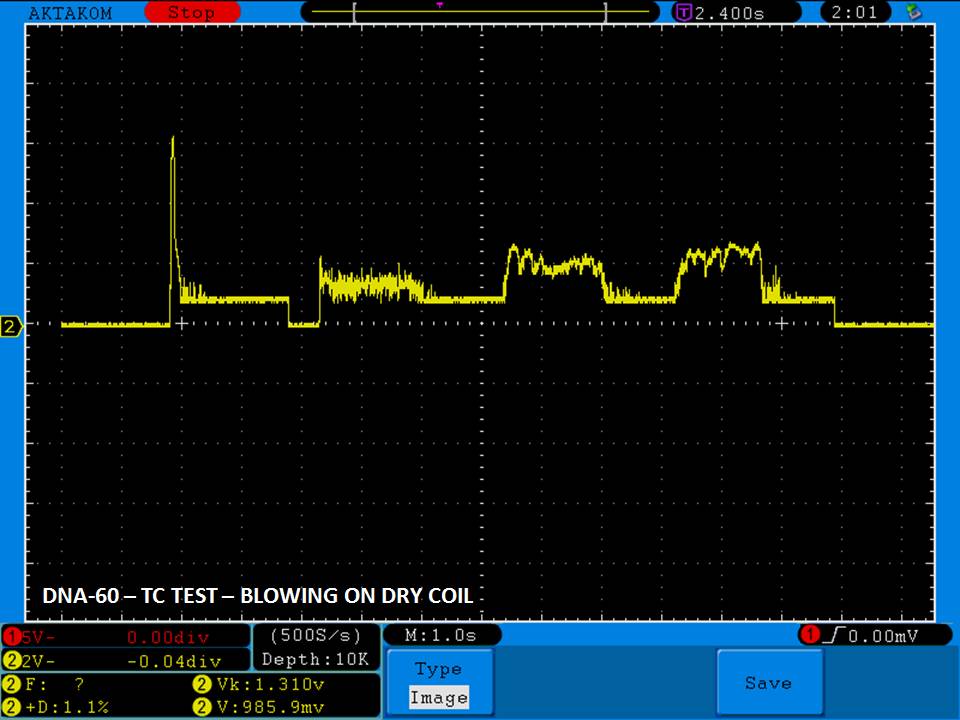
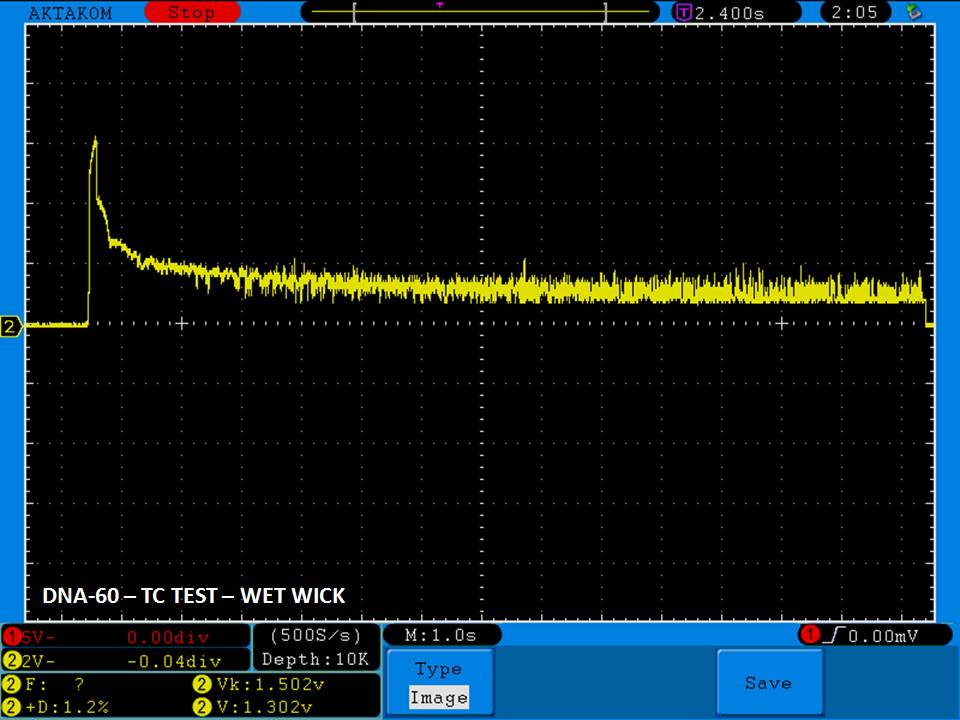
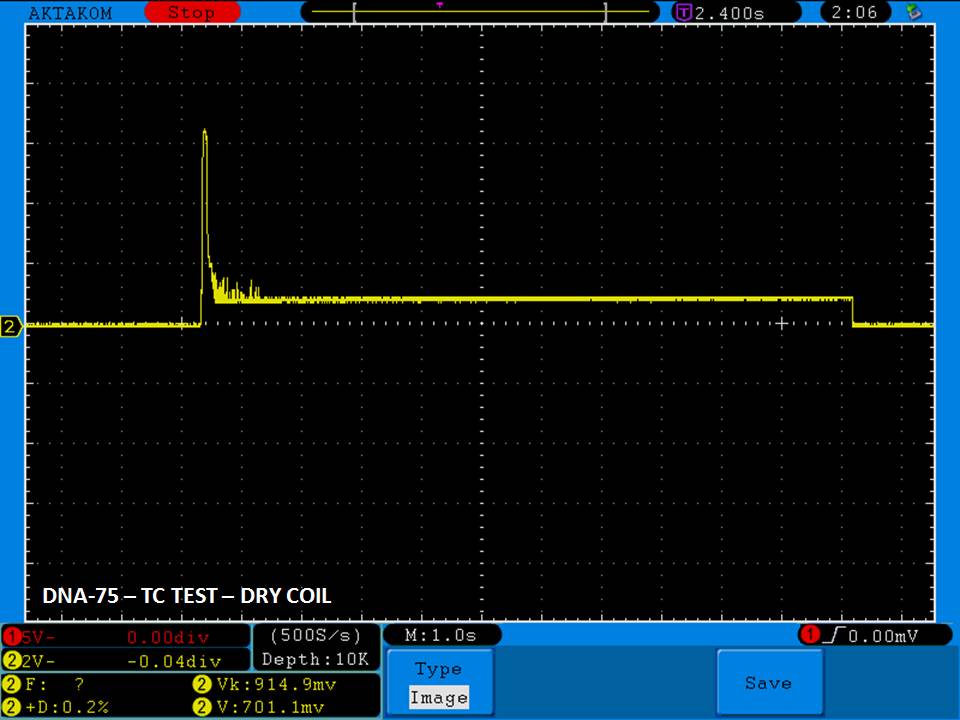
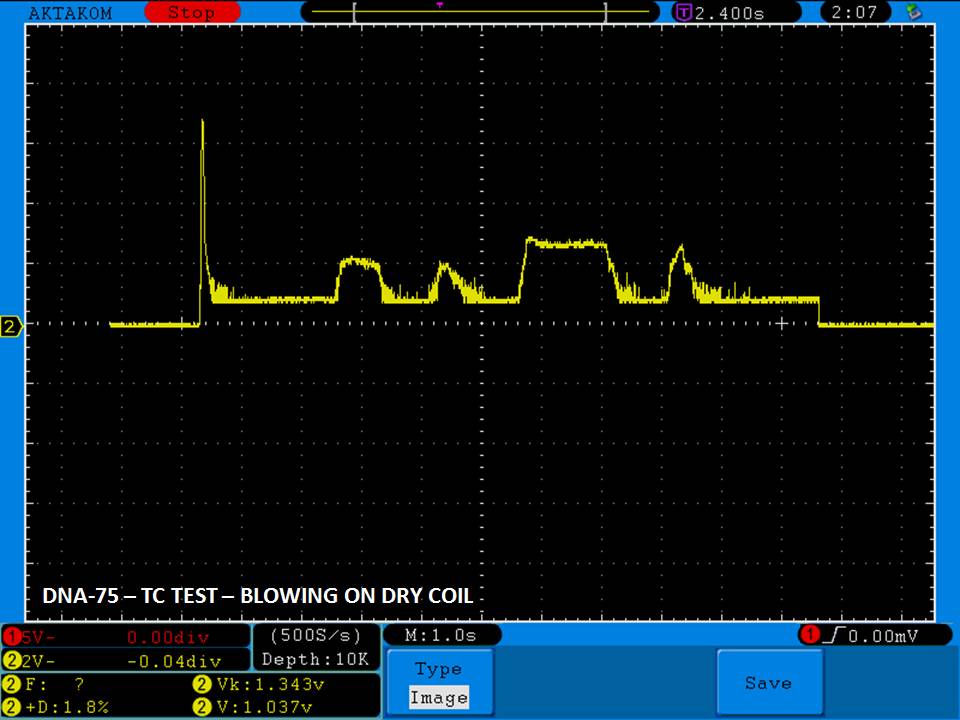
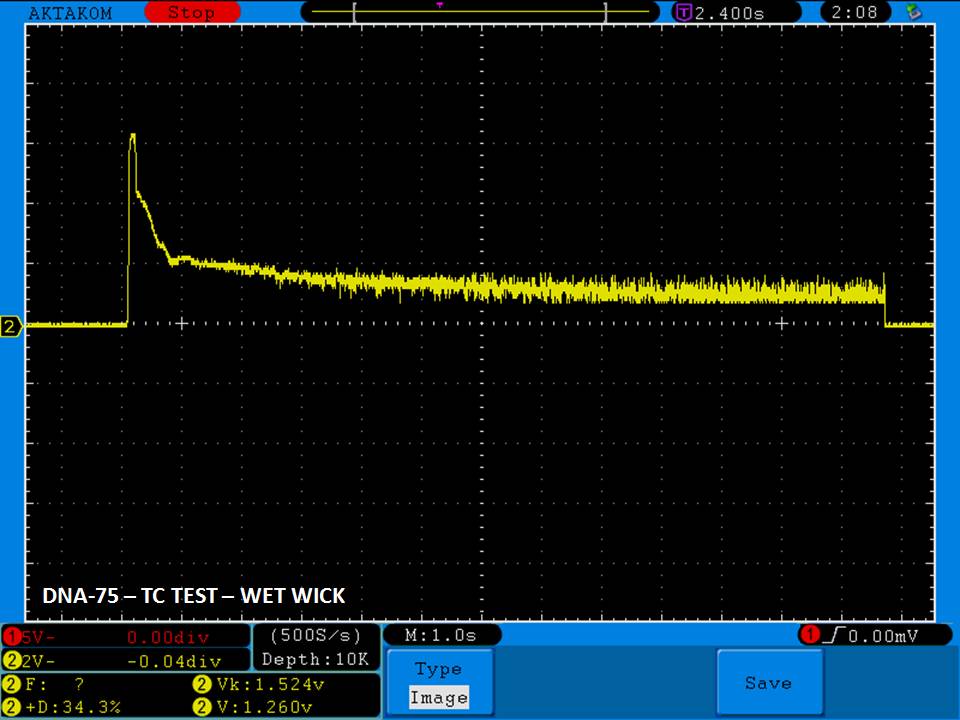
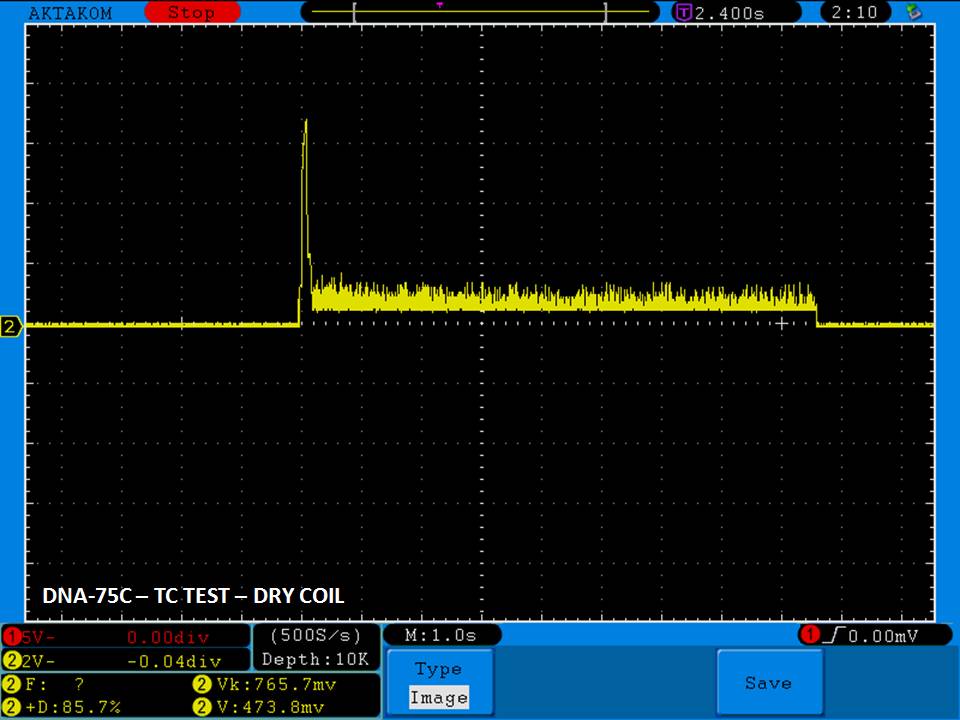
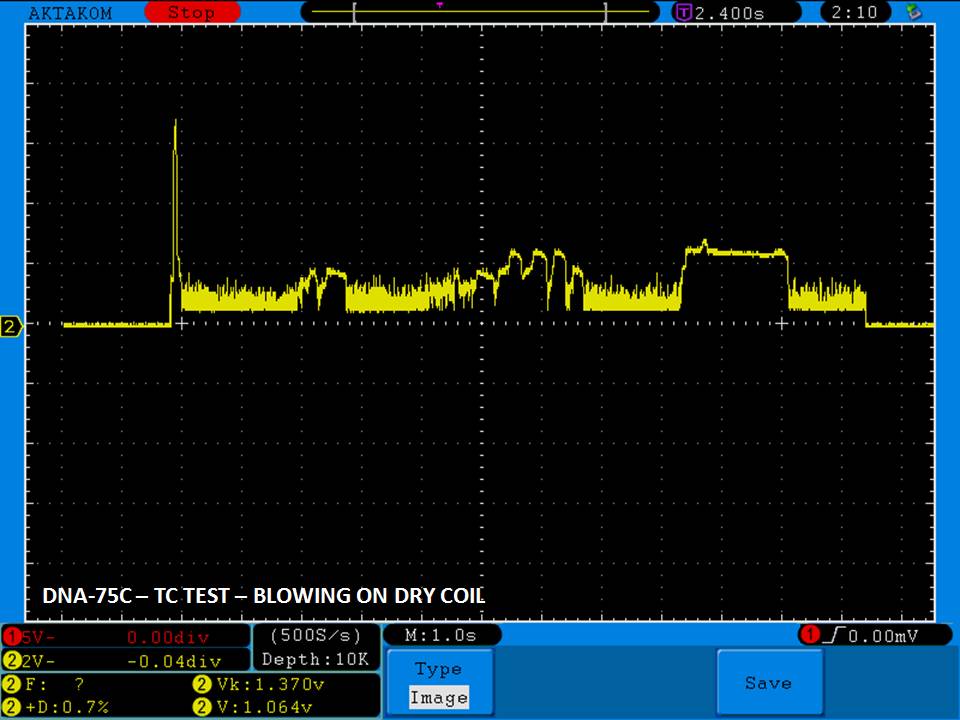
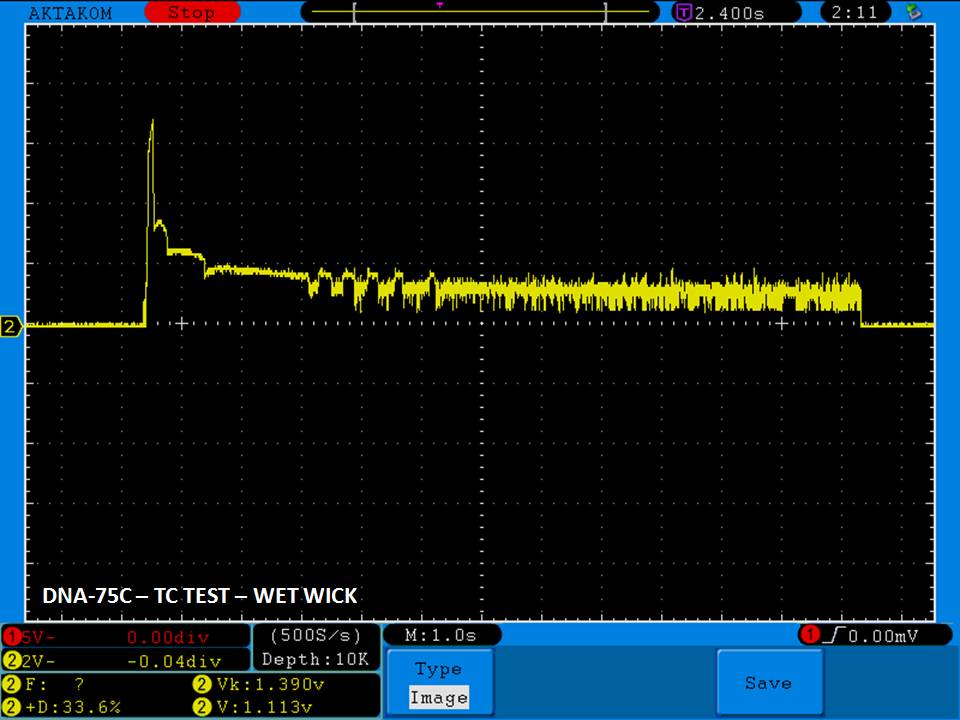
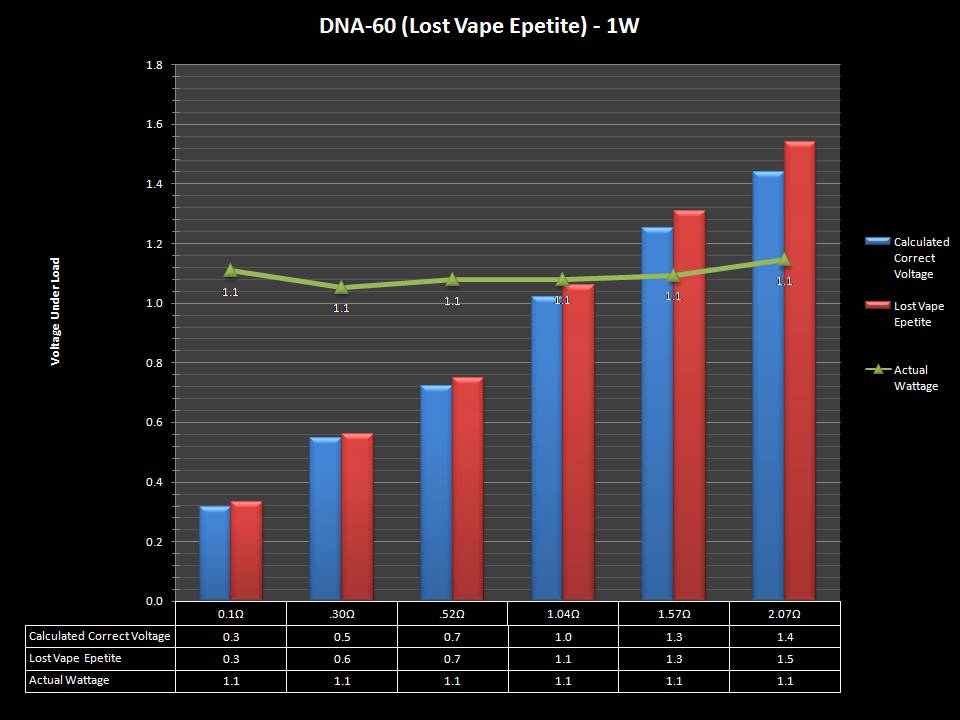
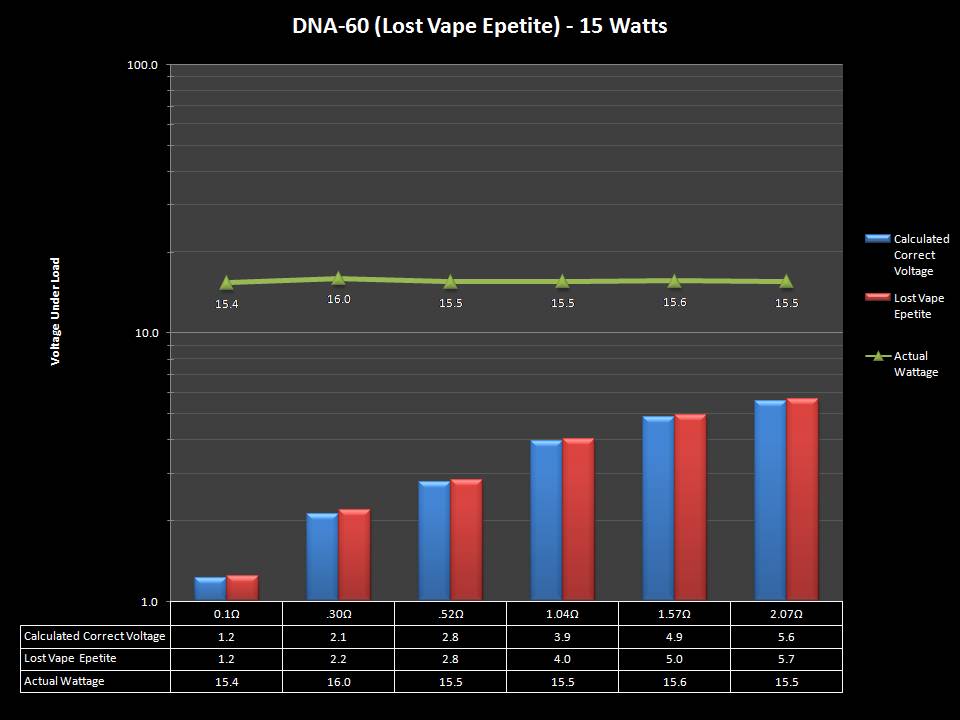
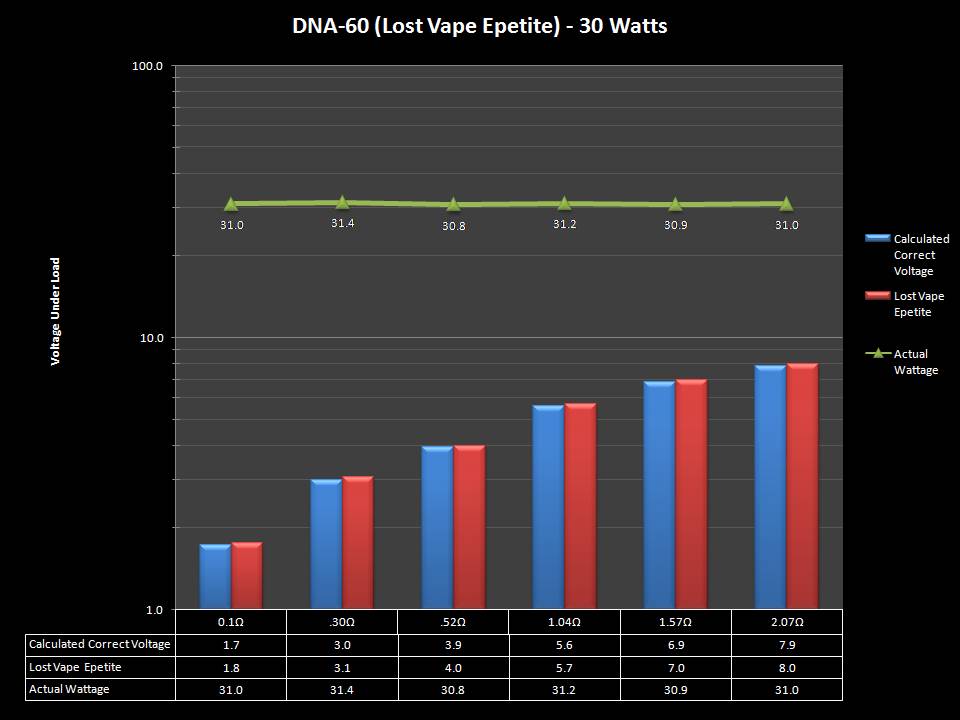
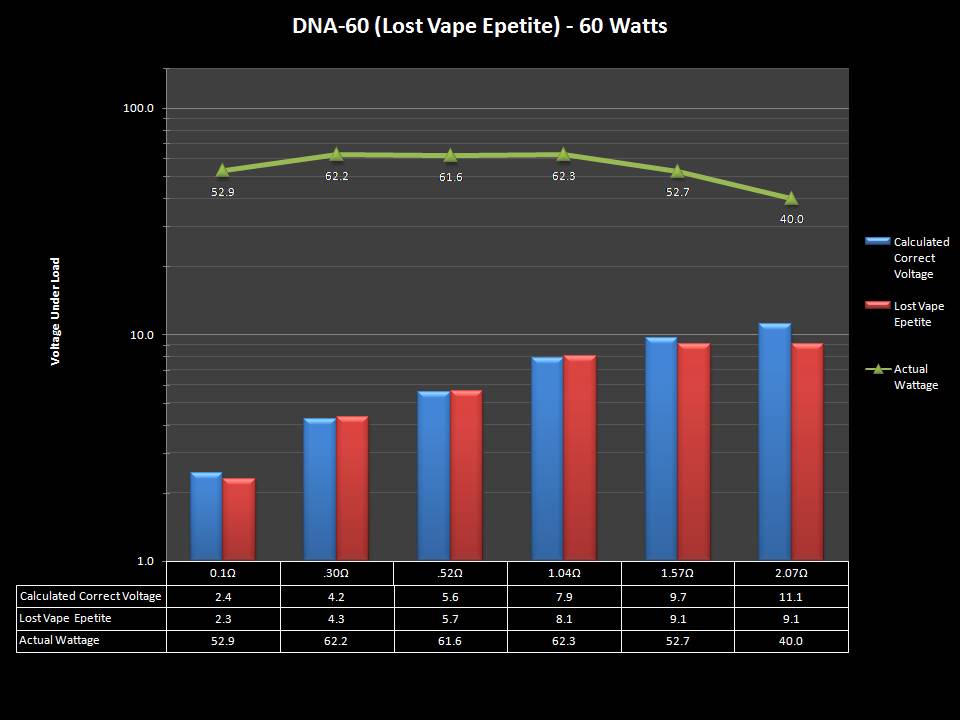
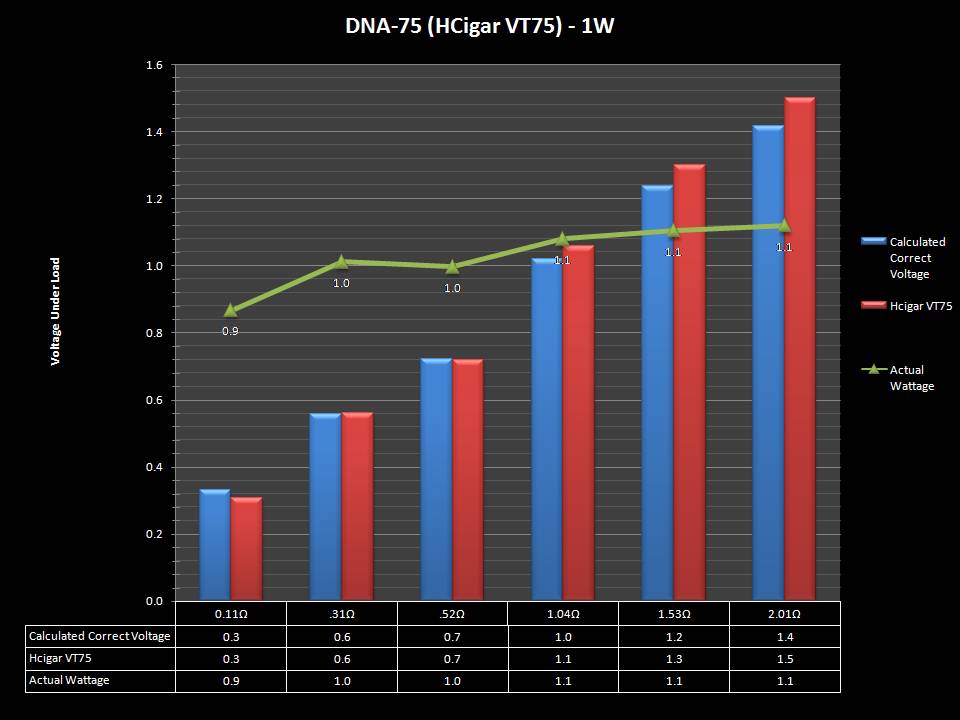
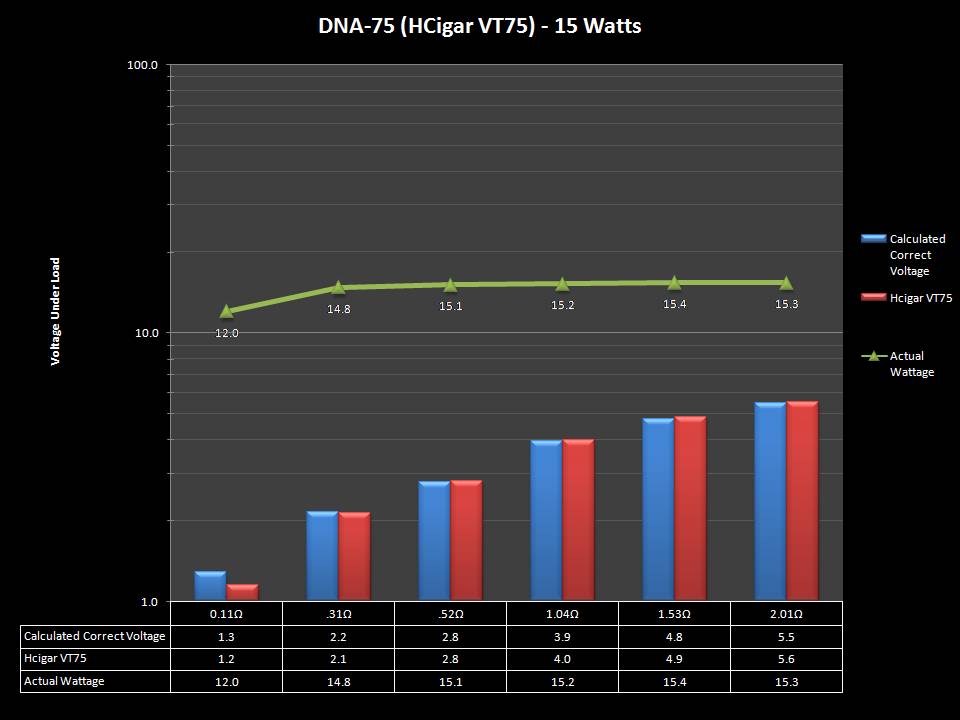
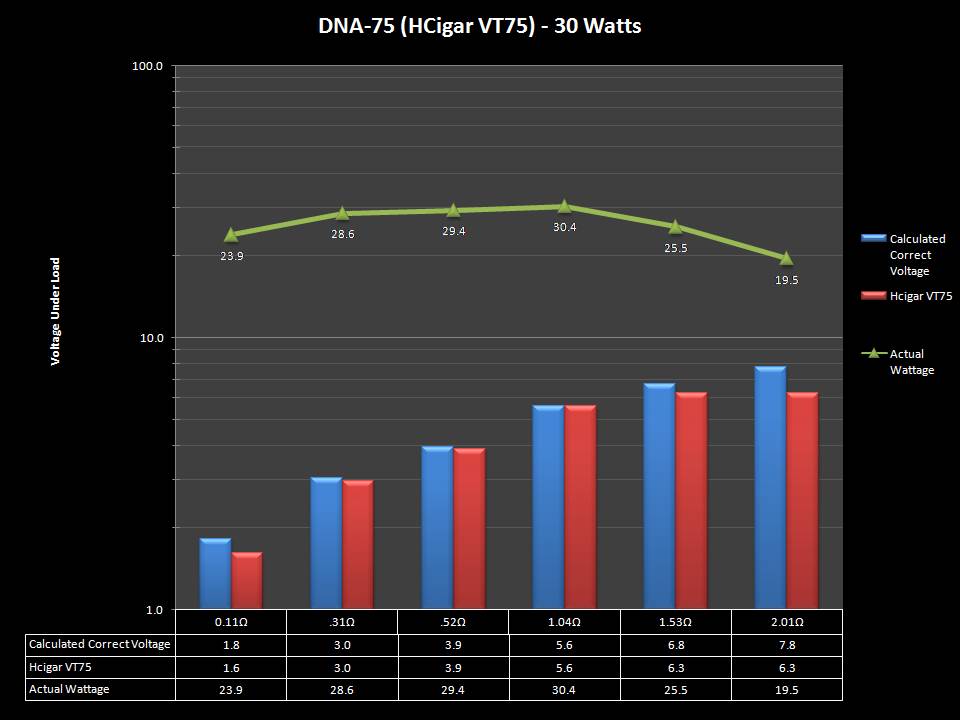
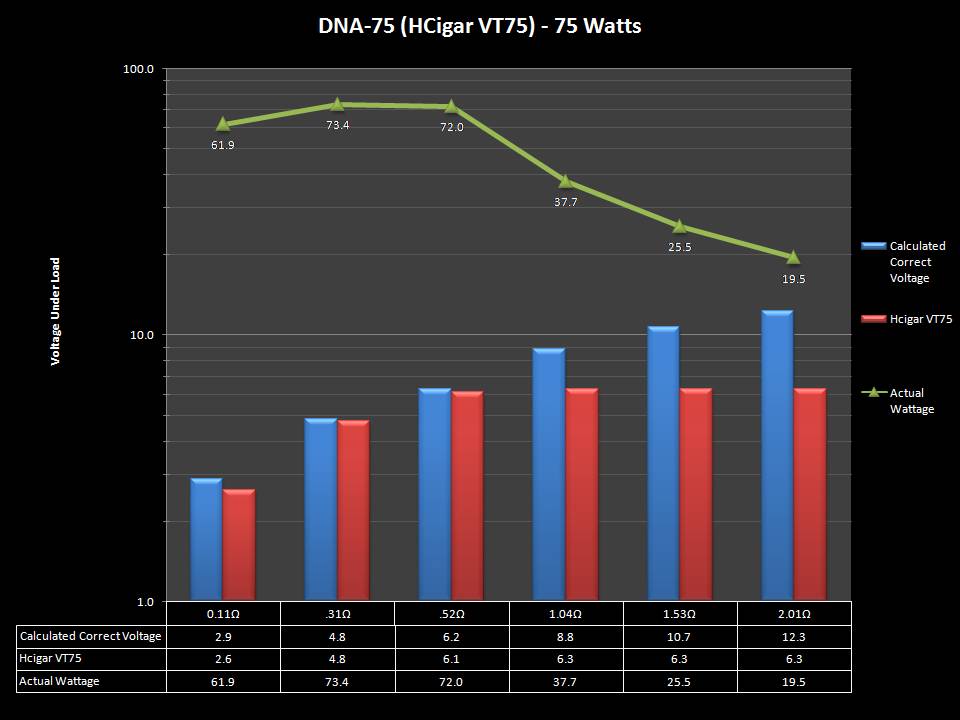
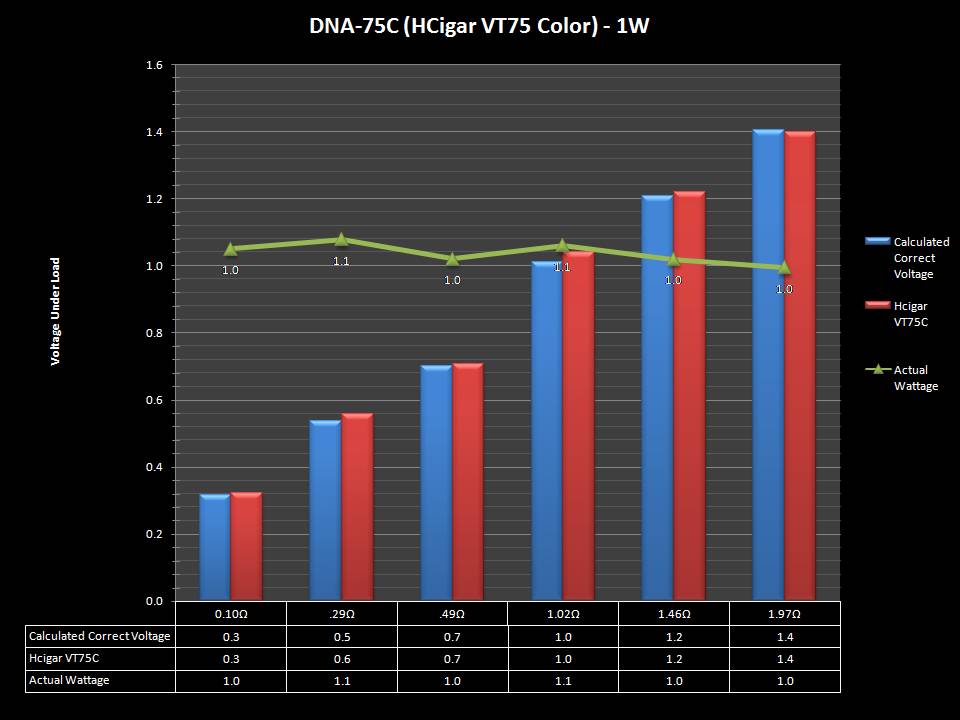
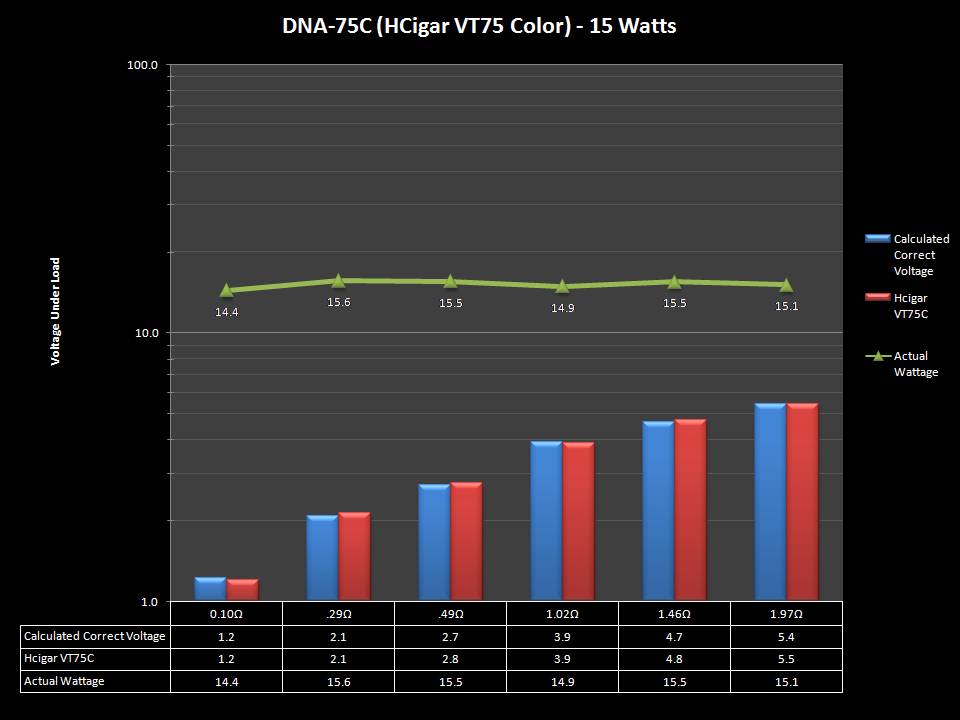
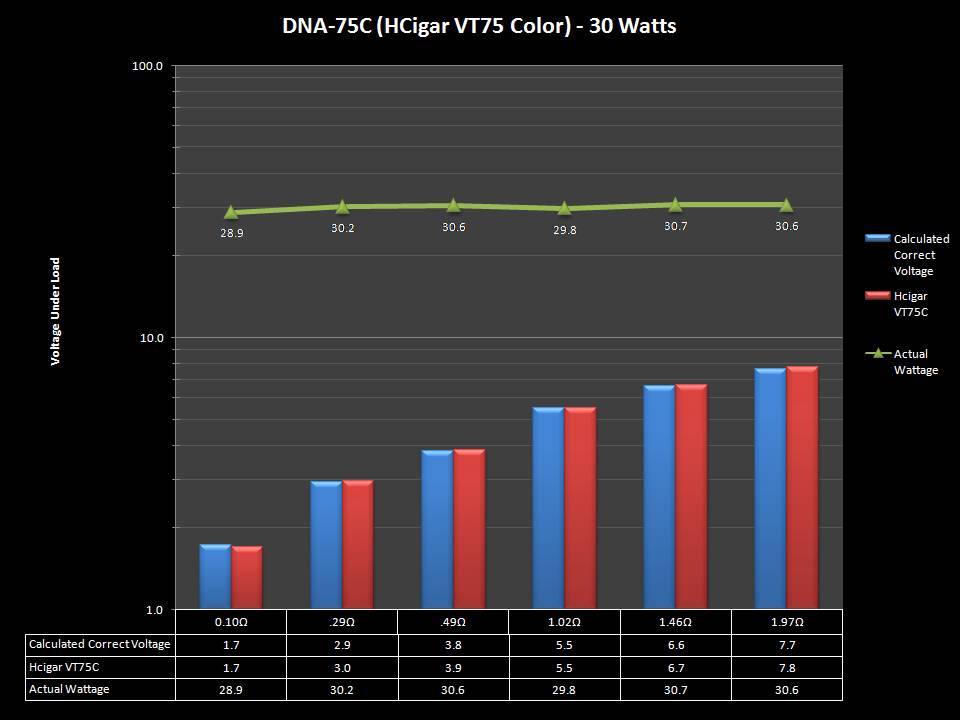
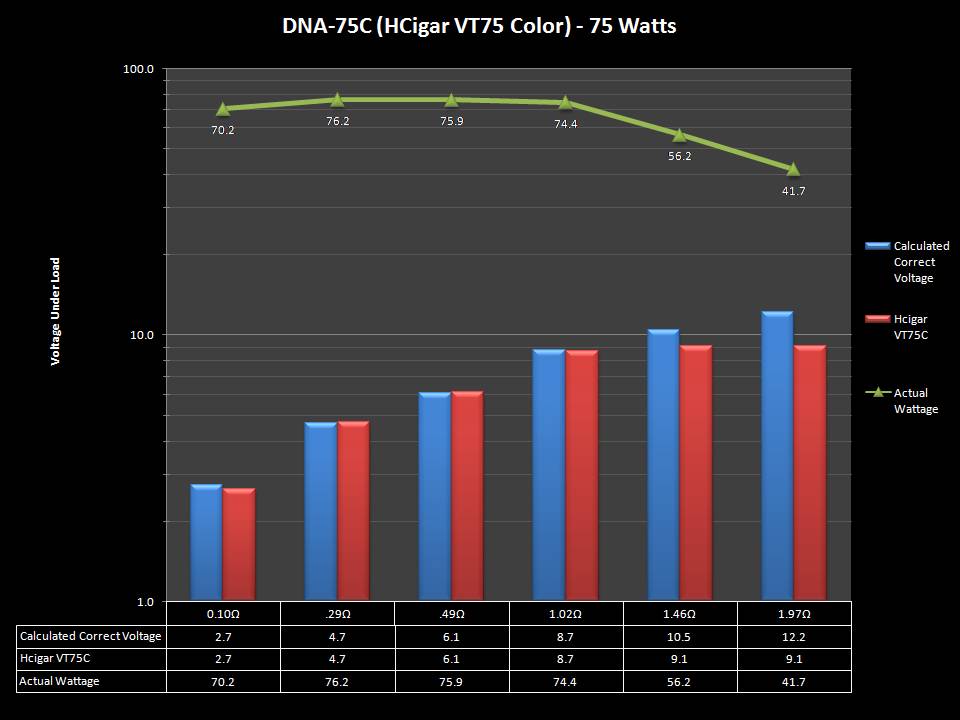
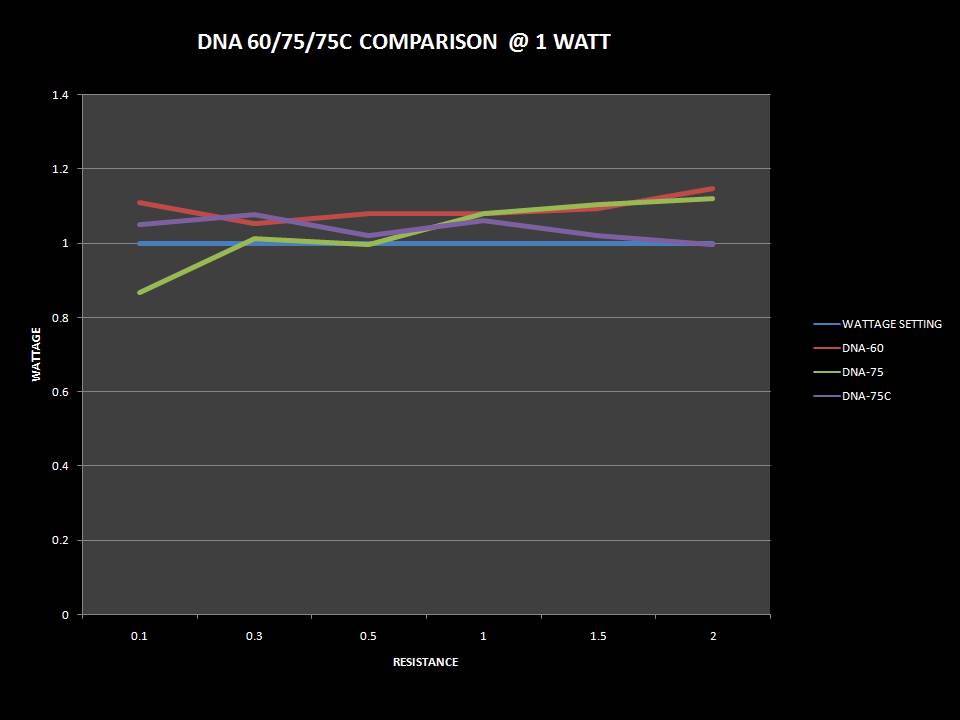
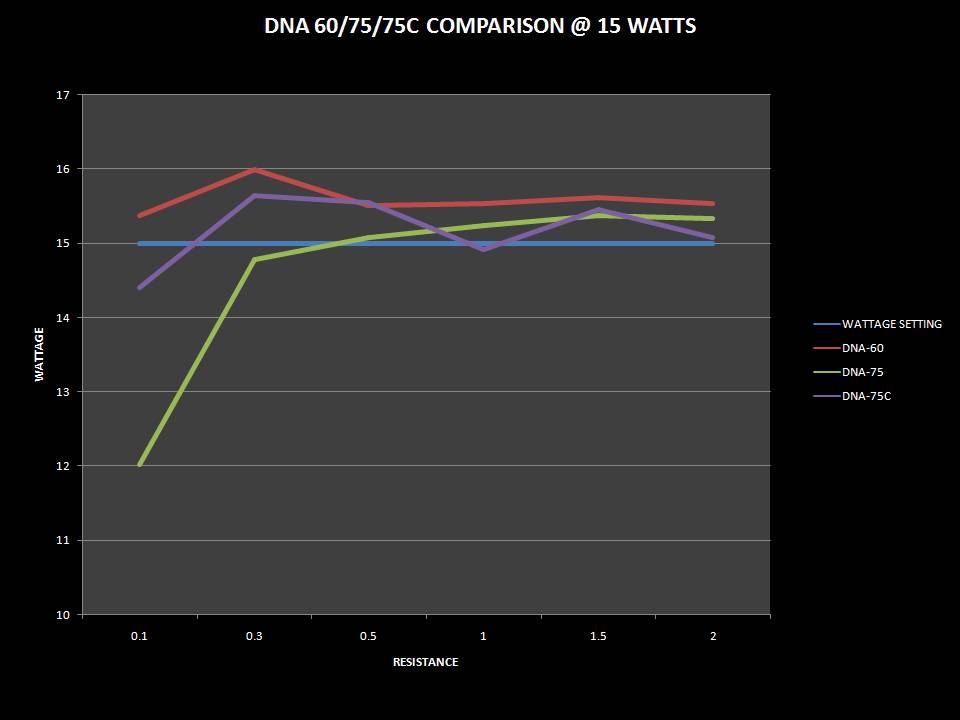
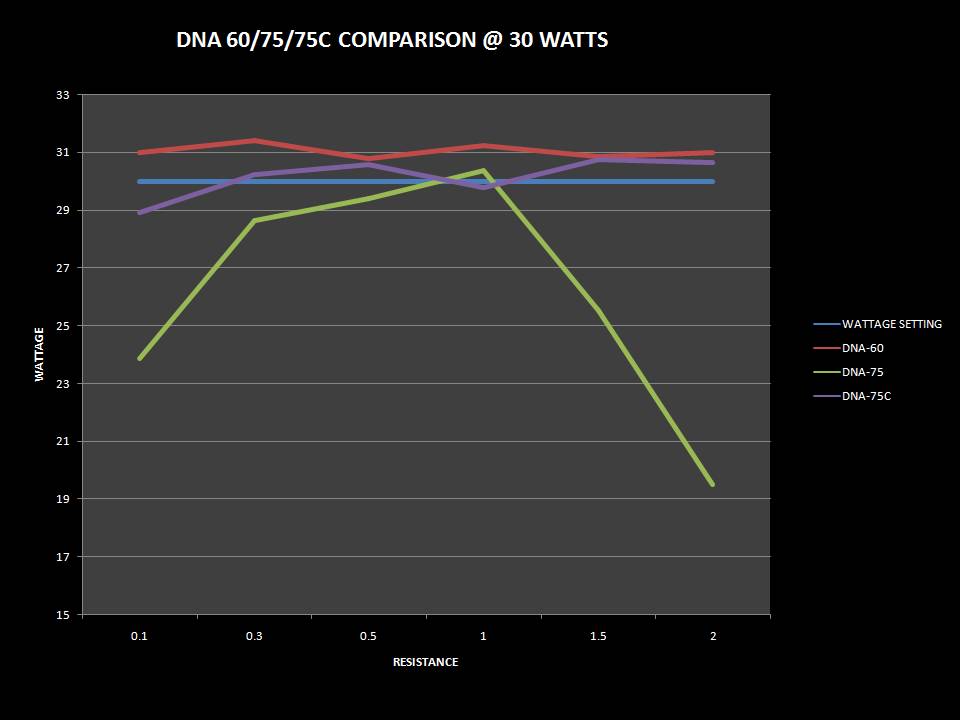
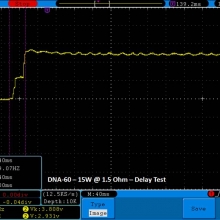
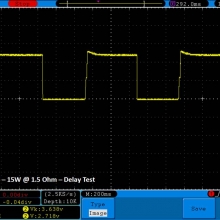
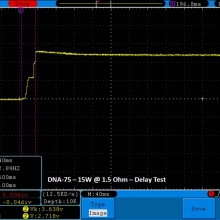
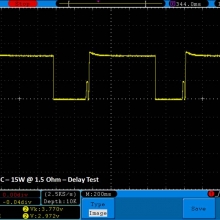
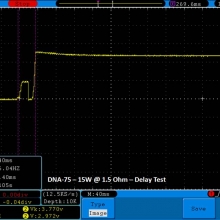
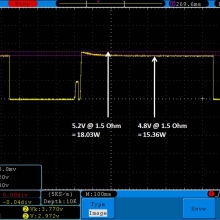
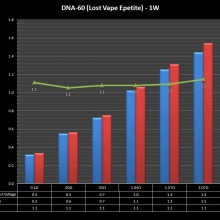
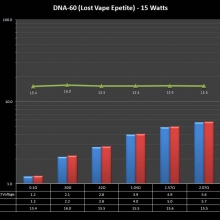
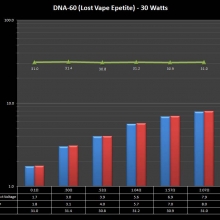
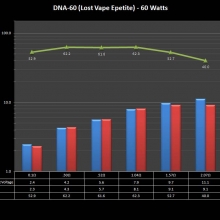
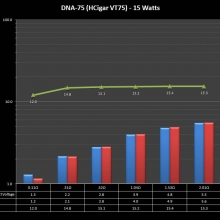
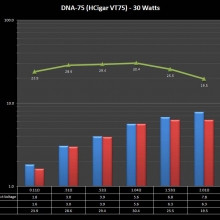
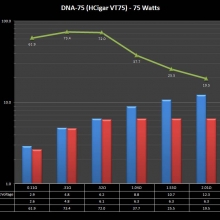
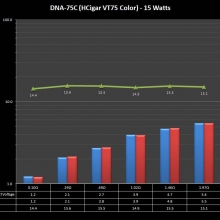
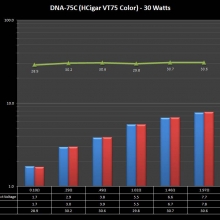
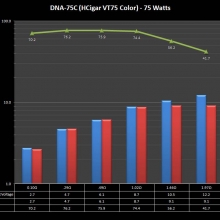
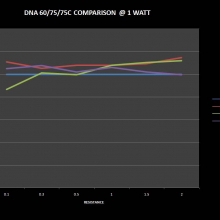
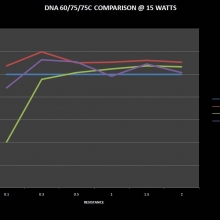
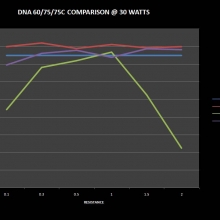
THE DNA-60, 75 & 75C COMPARISON & REVIEW
A PBusardo Review – The DNA-60/75/75C Comparison
We kick off this video by following up on the Sigelei Kaos LED test.
Then we…
- Compare the DNA-60, 75 & 75C boards.
- Take a look the DNA-75C default theme.
- See where to find and how to load new themes.
- See the differences in Escribe when using a DNA-75C
- Talk quickly about the devices used in the comparison
- Compare the numbers and signals of the DNA-60, 75 & 75C
- Do some thumbs up & down on the DNA-75C
The Links:
Evolv Homepage
Evolv Forum – Find Themes Here
VaporDNA
Lost Vape
HCigar
The Video:
*NOTE: Any use of these videos in part or in their entirety without Phil Busardo’s expressed written consent is strictly prohibited.
The Photos:
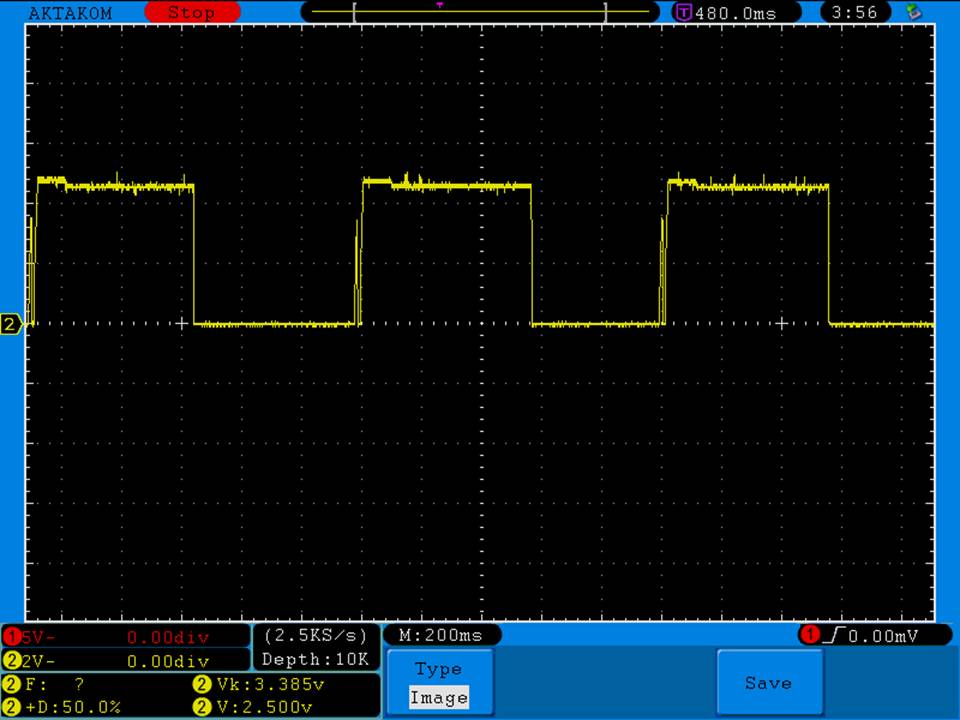
A BATTERY MOOCH POST: Bench Test Results: Efest 30A 3000mAh 20700…accurately rated, same cell as iJoy/Ampking
This cell has only one rating on the wrap, “Max continuous discharge = 30A” with a larger “30A” above that. I am delighted to say that the rating is accurate. It is also good to see that Efest took off the useless pulse rating that they originally had on an earlier version of the wrap.
I don’t know if they’ll carry this change in the way they rate their cells over to their 18650’s. I will be retesting all of the Efest 18650’s in a few weeks. We’ll see what happens.
This Efest appears to be the same cell as the iJoy and Ampking 20700’s. It uses a plastic top ring insulator though versus the paper insulator on the iJoy and Ampking. This might make a difference to those who often get e-liquid on the battery.
Three of the four Efest’s I tested didn’t perform quite as well as the iJoy/Ampking cells though. The Efest’s ran at a slightly lower voltage and with less capacity. The fourth Efest ran at a slightly lower voltage for a while but had the same capacity as the iJoy and Ampking.
I don’t know if the Efests were a slightly lower grade or if there are batch to batch differences for a particular grade of this cell. The differences in performance between these Efests and the iJoy/Ampking are minor though and I don’t think they will be noticeable in actual use.
This cell says “IMR 20700” on the wrap but I do not know if it actually uses the same lithium-manganese chemistry used by batteries from the big manufacturers with the IMR model number prefix.
I am rating this Efest at 30A and 3000mAh.
The four cells that were tested were donated by Efest (www.efestpower.com). Thank you!
Test results, discharge graph, photos: https://www.e-cigarette-forum.com/forum/threads/bench-test-results-efest-30a-3000mah-20700-accurately-rated-same-cell-as-ijoy-ampking.806303/
All my test results to date: https://www.e-cigarette-forum.com/forum/blog-entry/list-of-battery-tests.7436/
A BATTERY MOOCH POST: Never keep your batteries in a charger when traveling!
Whether it’s just to work or around the world, using a charger to store your batteries can lead to a fire or bursting of your battery if the wrap is damaged.
I feel this is what happened during a recent flight (photos from a CNN post). Two batteries, it appears, were being stored in an Efest SODA charger in a day pack during a flight. One of the batteries went into thermal runaway and caught fire. Luckily, everyone was ok.
Photos: https://imgur.com/a/mVYOy
What caused this?
It wasn’t because they were AWT batteries.
It wasn’t because the battery ignited on its own.
It wasn’t from being charged at the wrong rate.
It wasn’t from being used too hard.
Eagle-eyed vapers might notice that in the photos one of the two batteries is reversed. Could this be the cause?
No.
I tested this in a SODA charger at both charge rate settings and there were no problems.
But, like many chargers, if the wrap is damaged and the battery slips a bit out of position the positive contact of the charger can short-circuit the battery center positive contact to the negative outer ring at the top.
This can force the battery into thermal runaway leading to a fire or even violent bursting of the battery.
NEVER keep your batteries in the charger when traveling. Yes, it saves space. But it’s not worth the risk, especially when all the jostling could take an otherwise ok wrap and damage it.
ALWAYS store your batteries in a case, sleeve, or even a box and not in the charger.
ALWAYS keep your battery wraps and the insulator ring at the top of the battery in perfect condition.
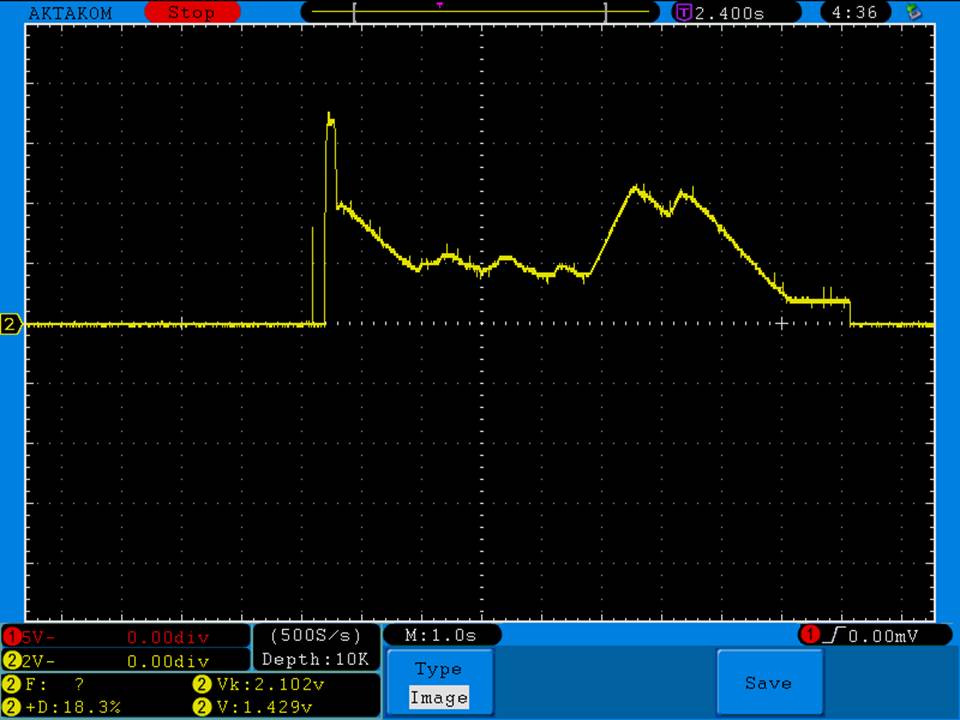
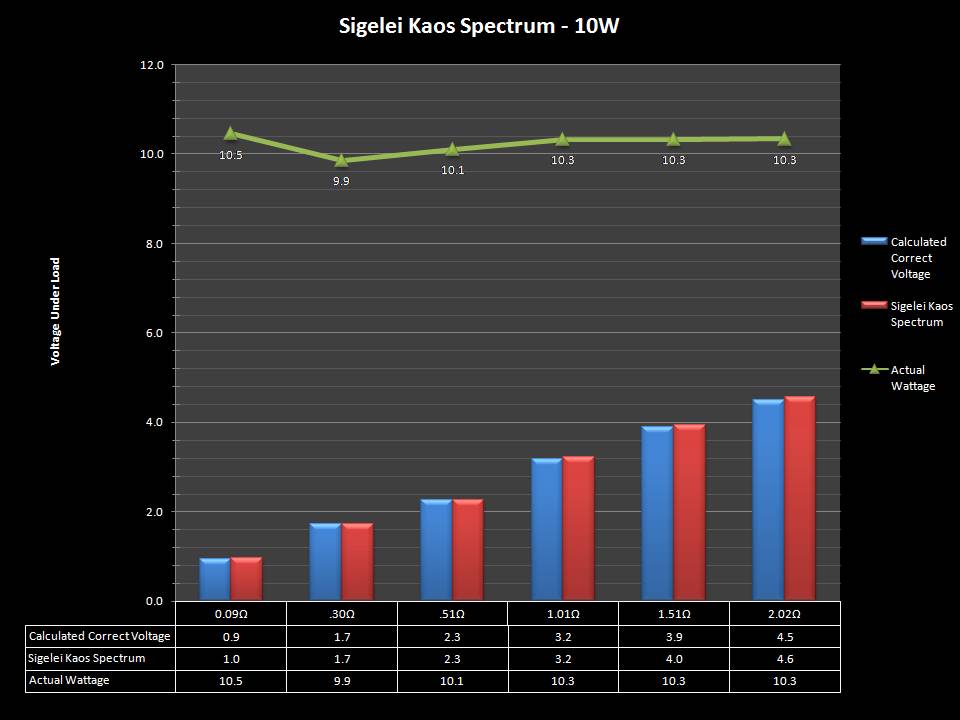
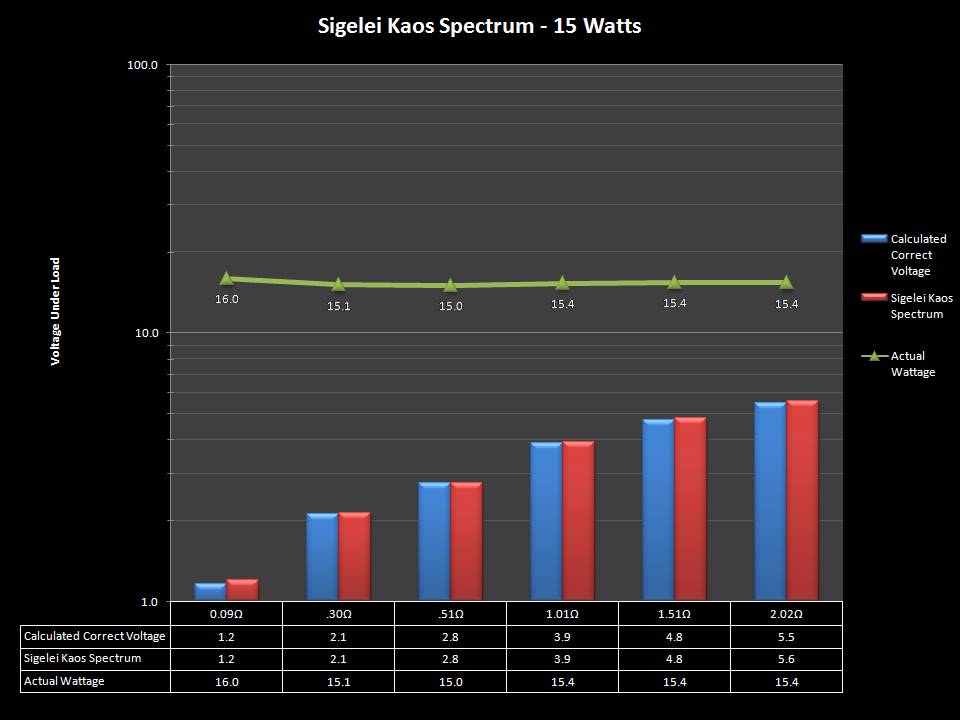
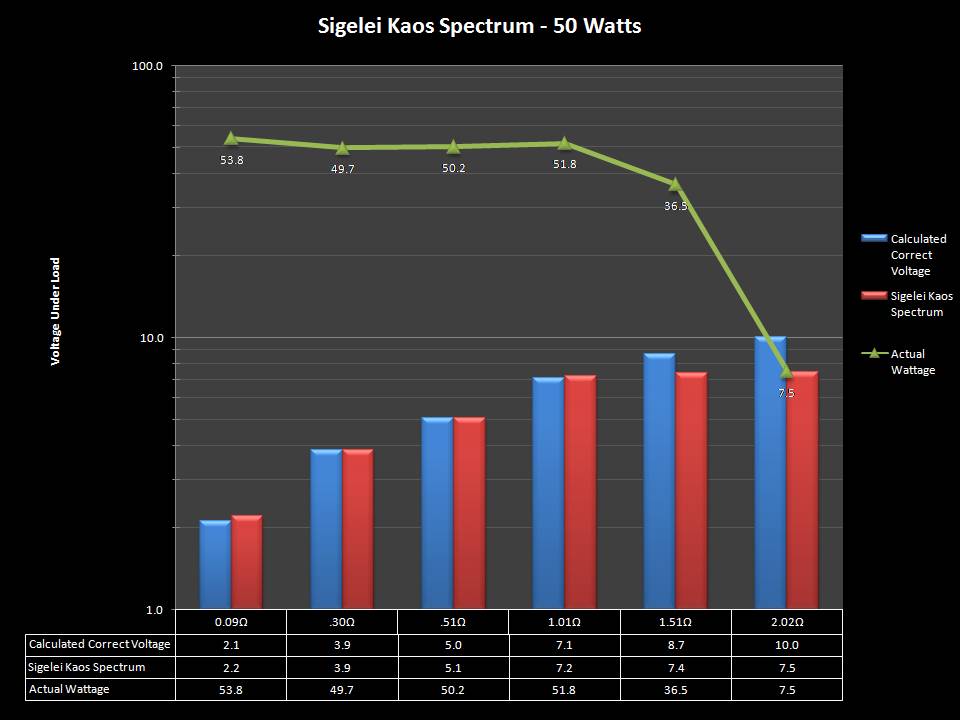
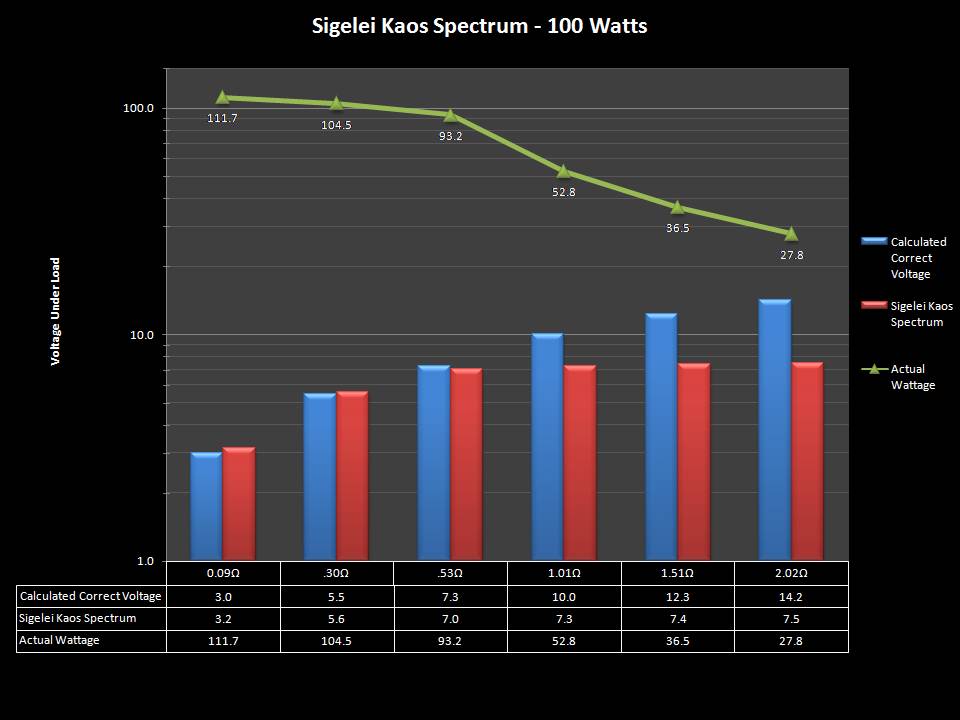
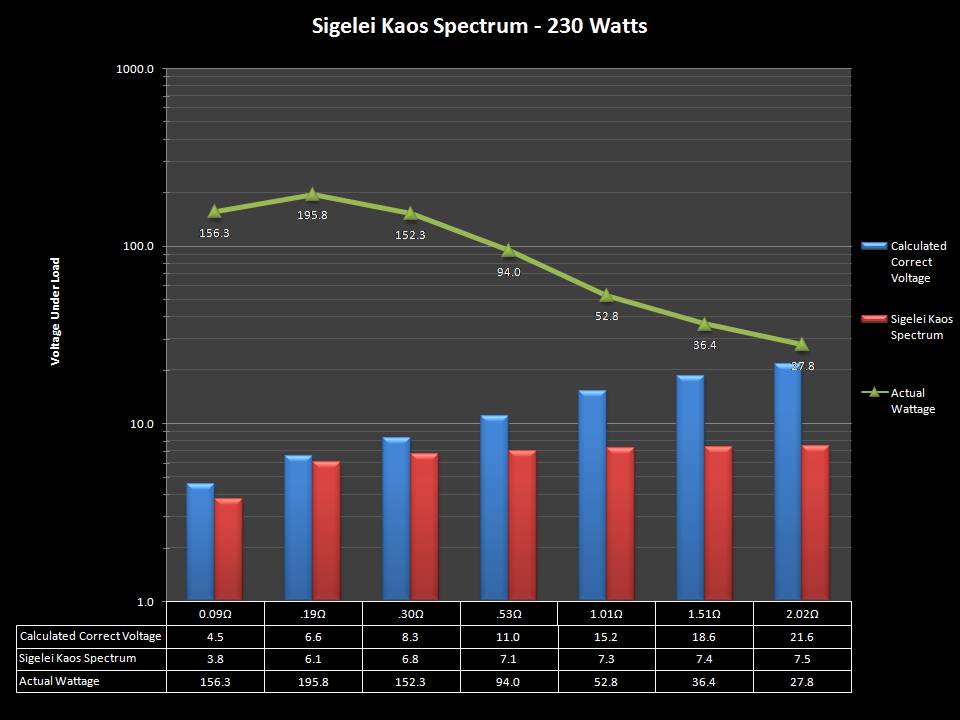
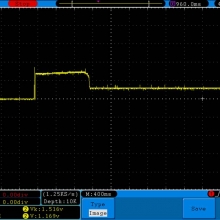
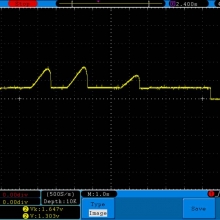
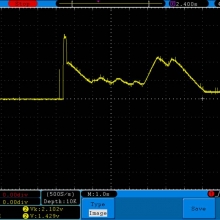
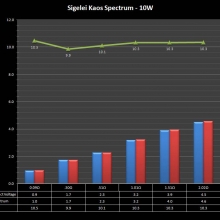
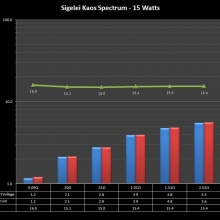
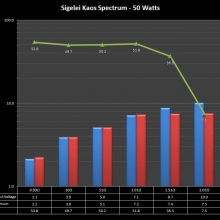
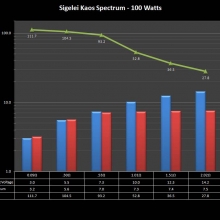
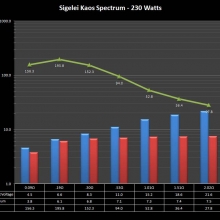
THE SIGELEI KAOS SPECTRUM
A PBusardo Review – The Sigelei Kaos Spectrum & Last “Not A” Contest Winner
In this video we take a look at the Sigelei Kaos Spectrum and find out who won the last “Not A” Contest.
The Links:
Sigelei
VaporDNA
Aspire
Eleaf
Freemax
NexVap
Smith & Baxter
Halcyon
The Video:
*NOTE: Any use of these videos in part or in their entirety without Phil Busardo’s expressed written consent is strictly prohibited.
The Photos:
10% OFF @ MTLVAPORS.COM – USE COUPON CODE “switch”!!
A BATTERY MOOCH POST: Nitecore 40A 4200mAh 26650 Bench Test Results…useless pulse rating, performs same as others
This cell has multiple ratings on the wrap, making it quite confusing as to what its actual ratings are. These include “Discharge Current: 40A”, “DISCHARGE CURRENT: 21A/40A”, and “40A”.
I am assuming that 21A is the continuous current rating but it also has a 40A (pulse?) rating in multiple places on the wrap. This is unacceptable as that 40A rating cannot be used to compare this cell to any other. It is useless.
This Nitecore appears to be the same cell used for the Basen, Brillipower, HohmGrown 4200mAh-4500mAh cells and it performs like the others.
The wrap is not the standard heat shrink plastic. It is self-adhesive and appears to be similar to Mylar. I do not know how durable it is compared to the wraps being used now by other companies.
This cell says “IMR26650” and “High Drain Li-Mn” on the wrap but I do not know if it actually uses the same lithium-manganese chemistry used by batteries from the big manufacturers with the IMR model number prefix.
I am rating this Nitecore at 23A and 4200mAh.
The two cells that were tested were donated by IMRBatteries (www.imrbatteries.com). Thank you!
Test results, discharge graph, photos: https://www.e-cigarette-forum.com/forum/threads/nitecore-40a-4200mah-26650-bench-test-results-useless-pulse-rating-performs-same-as-others.805473/
All my test results to date: https://www.e-cigarette-forum.com/forum/blog-entry/list-of-battery-tests.7436/




























































































































































































 Store
Store












