Category: Recent News
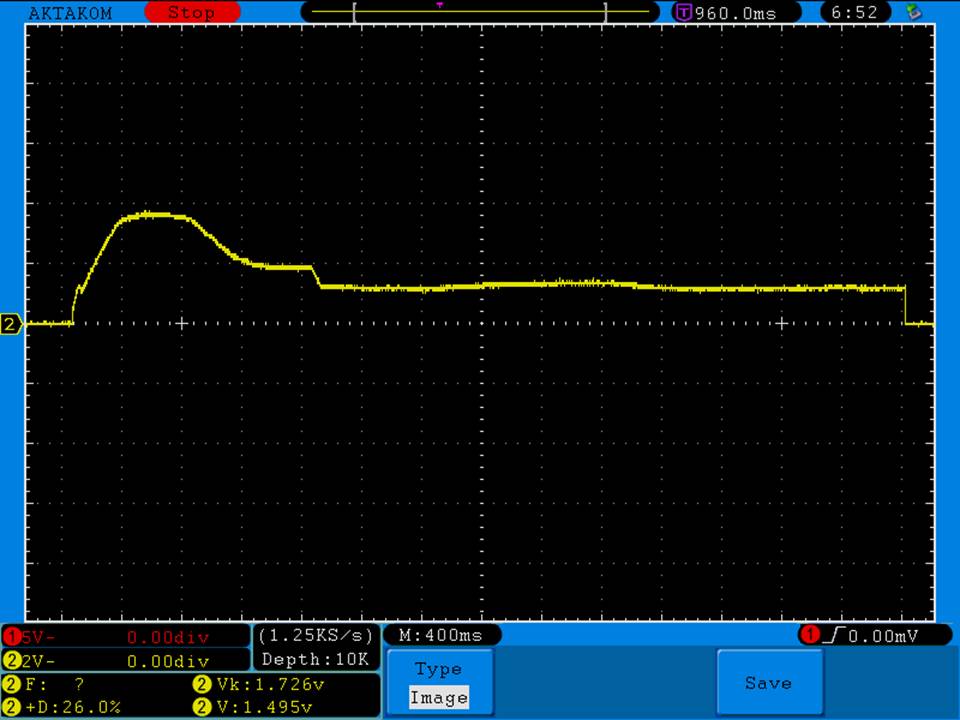
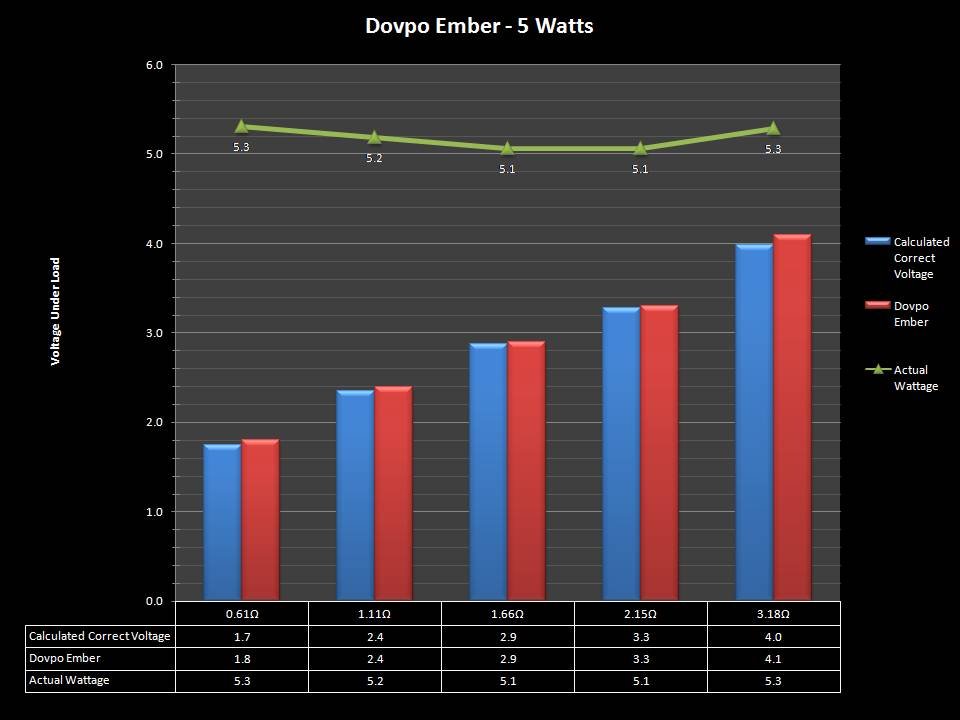
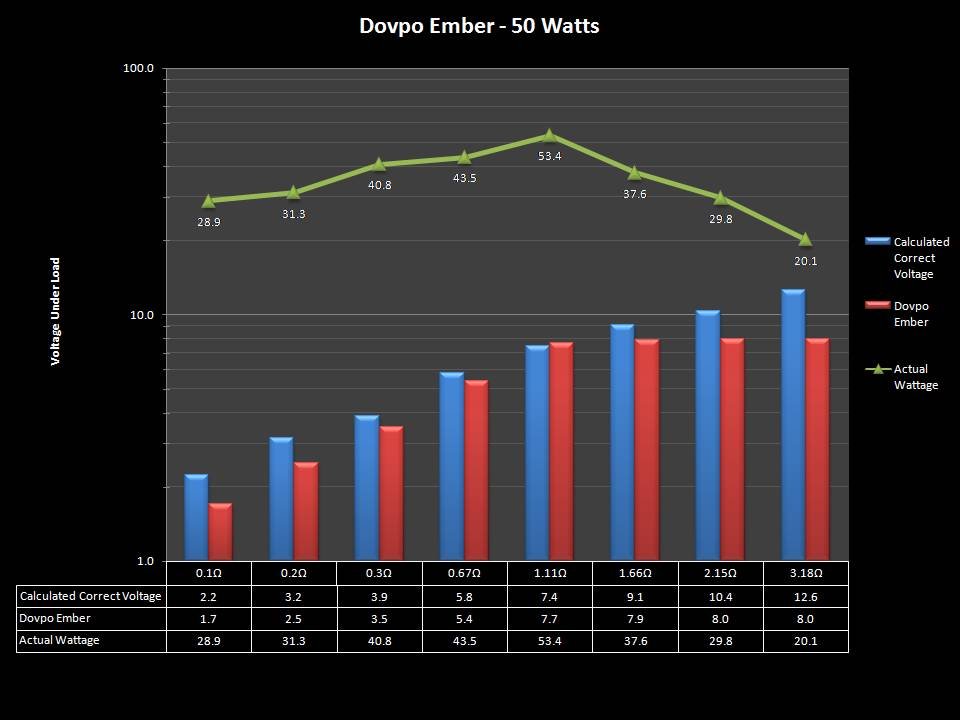
THE “MINI MARATHON” CONTINUES WITH THE DOVPO EMBER
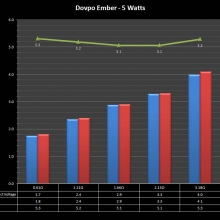
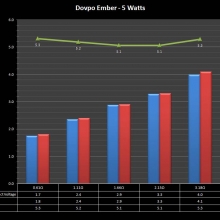
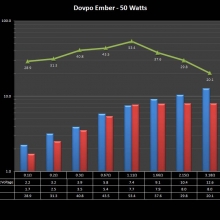
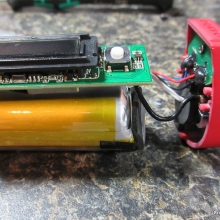
A PBusardo Review – The Mini Marathon Part 5 – The Dovpo Ember
In this episode of the “Mini Marathon” we take a look at the Dovpo Ember.
The Links:
Dovpo
Big Marvel
The Video:
*NOTE: Any use of these videos in part or in their entirety without Phil Busardo’s expressed written consent is strictly prohibited.
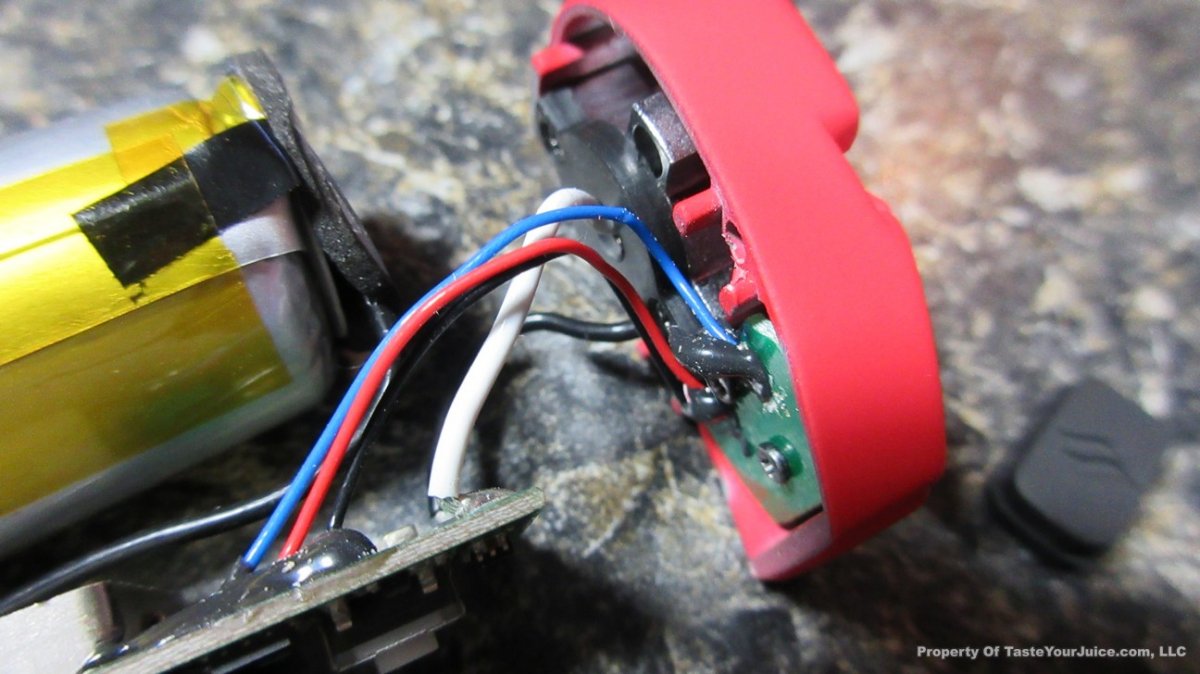
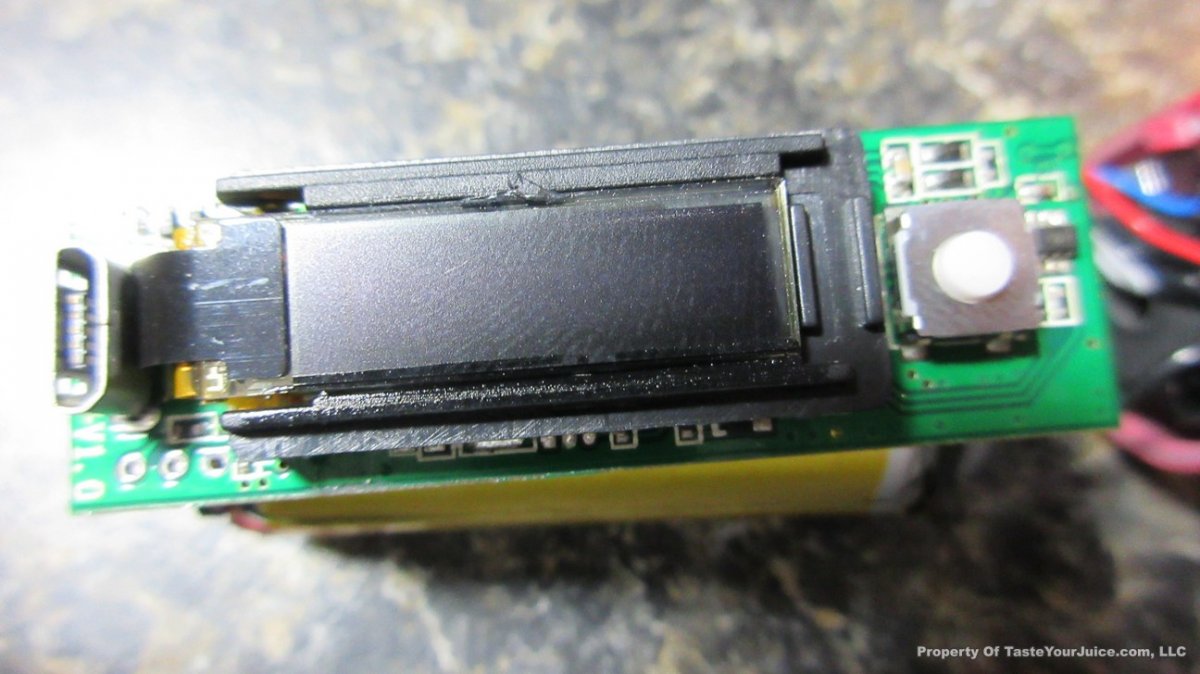
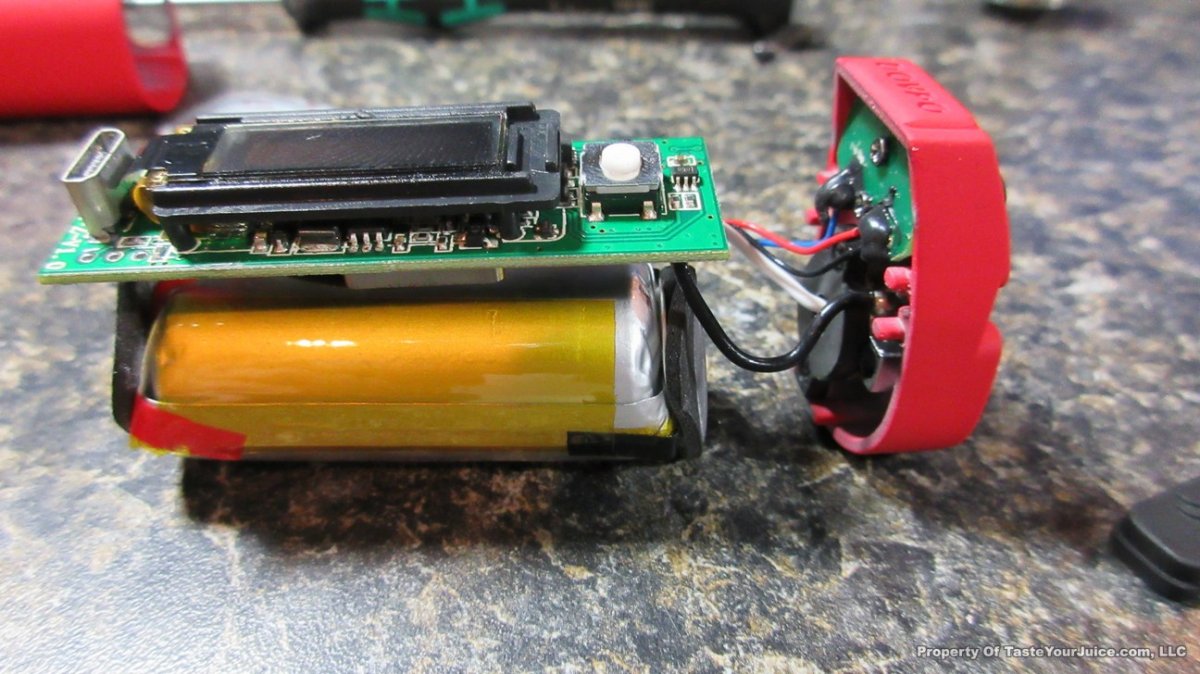
The Photos:
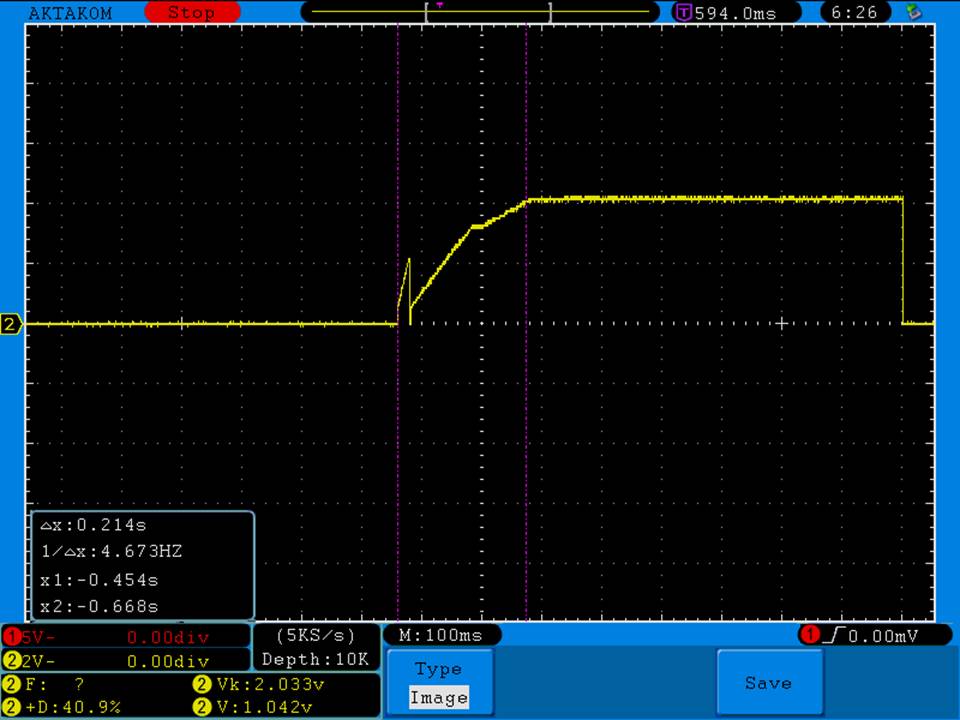
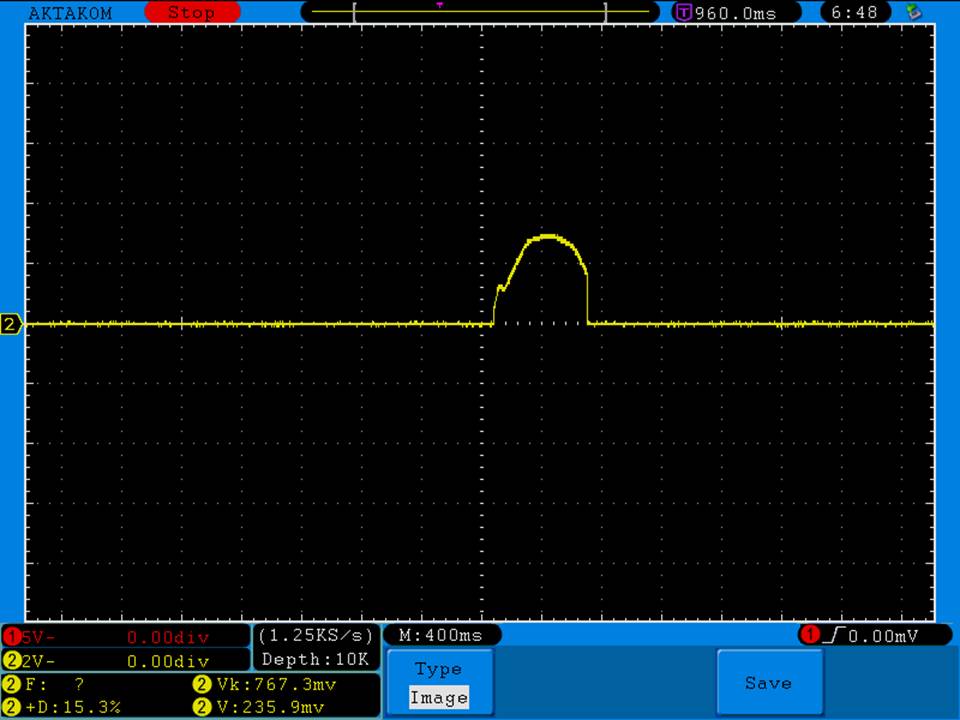
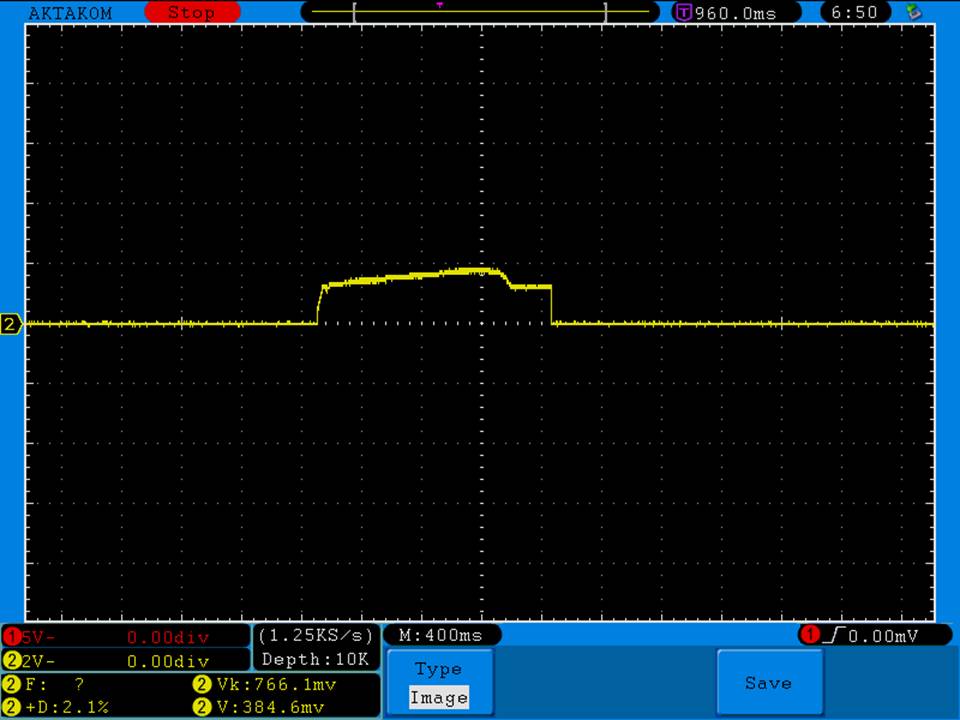
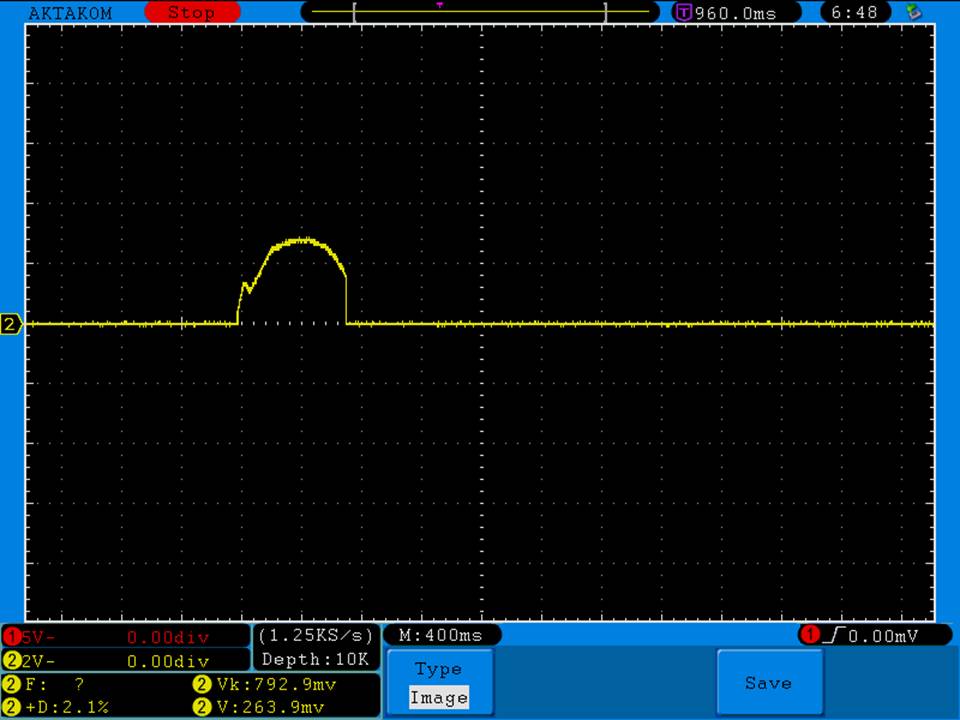
A BATTERY MOOCH POST: Busbars 25A 1750mAh 18650 Bench Test Results…accurately rated 25A, fantastic battery!
Bottom Line: This Busbar cell is a great performer and is accurately rated at 25A. I gave it an 1800mAh capacity rating, a bit higher than the claimed 1750mAh. It lists both the continuous and pulse ratings on the wrap and is the first cell to also list the pulse length and duty cycle for the pulse rating! This is fantastic and is a great step towards completely open and accurate cell ratings, thank you. The criteria for the pulse rating isn’t known (temperature? cycle life? voltage sag?) but the voltage at 50A pulsed is good and is comparable to, or better than, other cells rated around 25A.
It’s supposed to be a “hybrid” chemistry cell (sometimes called INR), but its discharge curves are much more like an ICR cell than other hybrid cells like the 25R and VTC4/5. Like an ICR its voltage holds pretty steady before quickly dropping as the cell approaches empty. I haven’t directly compared Busbars against the Tesiyi 40A 2600mAh or Aspire 40A 1800mAh ICR cells but hope to do so soon.
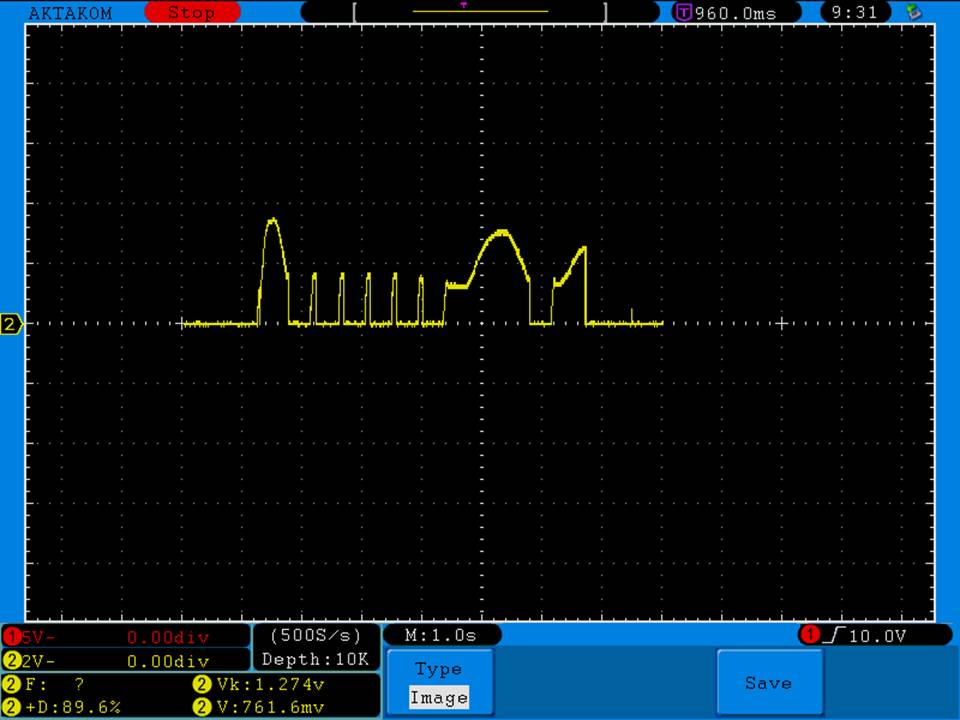
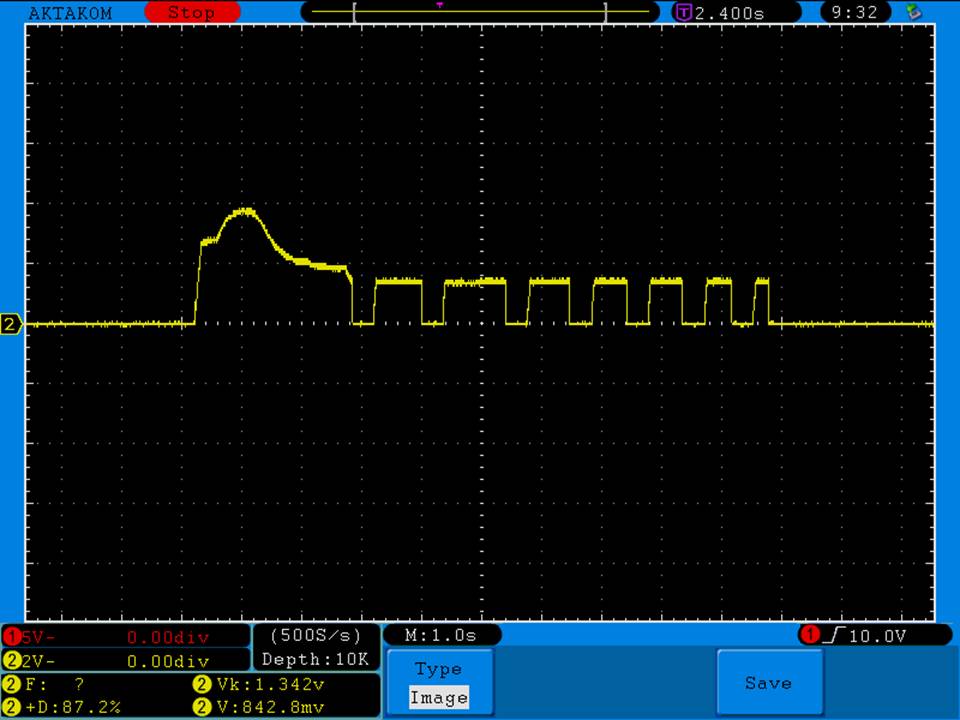
Test results, discharge graph, photos: https://www.e-cigarette-forum.com/forum/threads/busbars-25a-1750mah-18650-bench-test-results-accurately-rated-25a-fantastic-battery.749931//
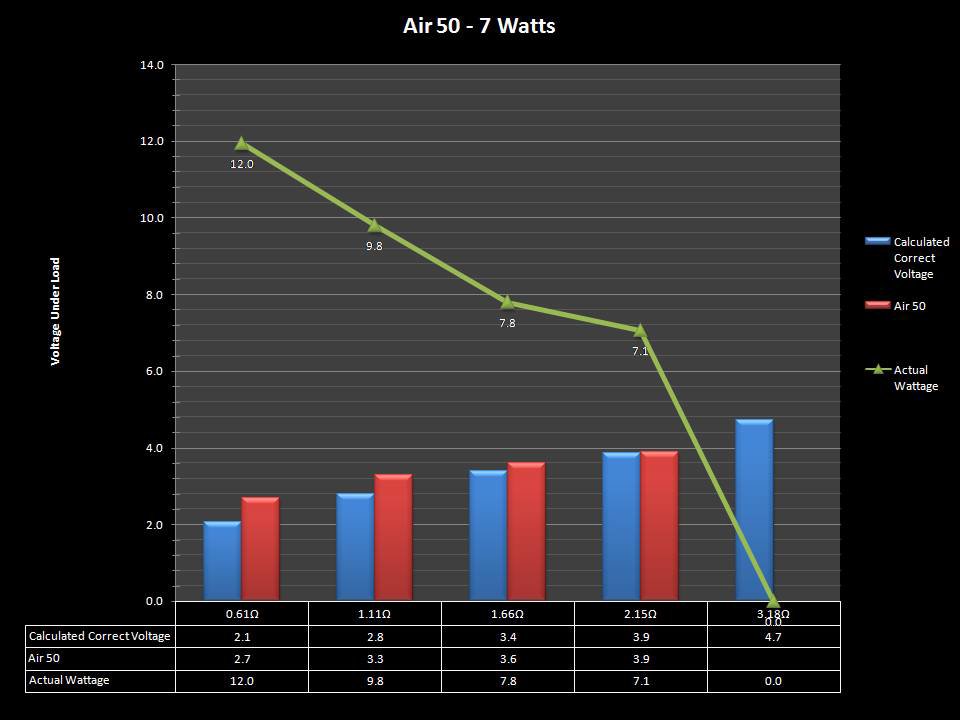
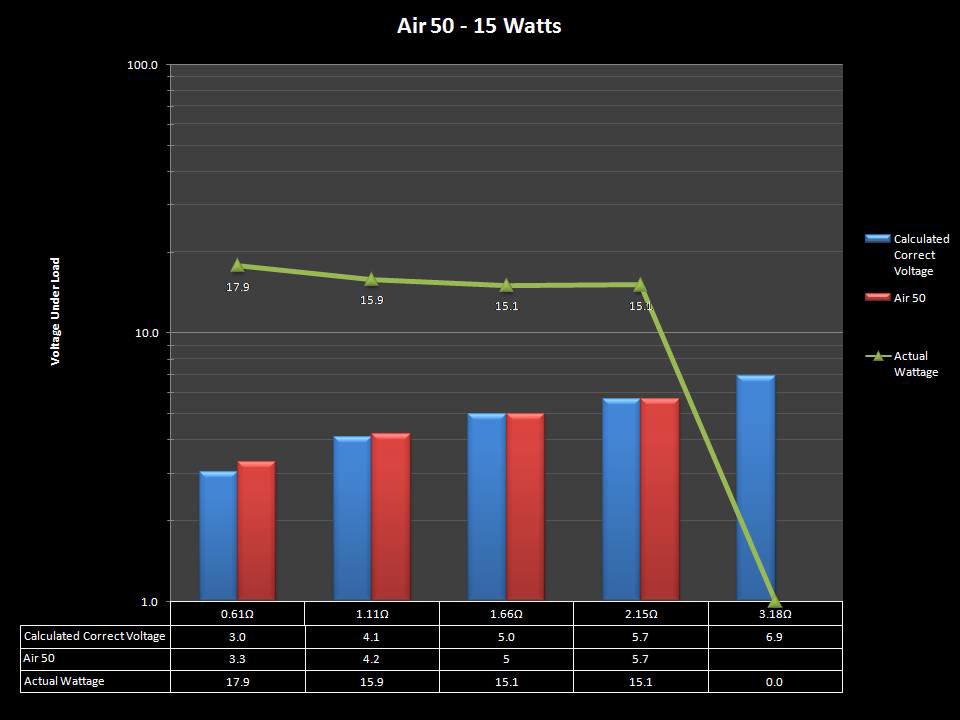
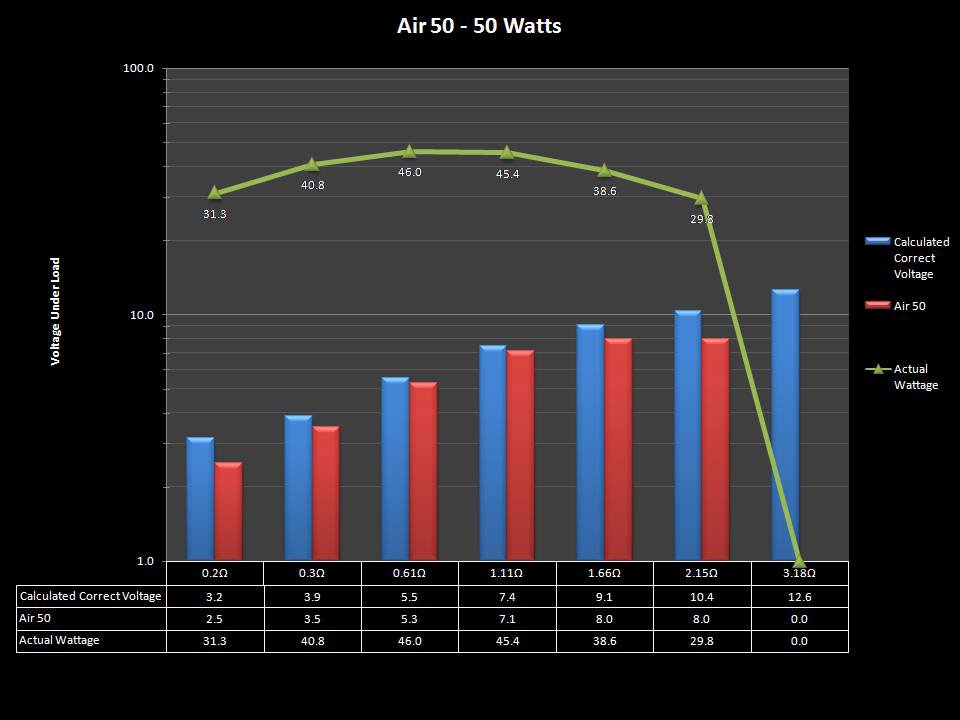
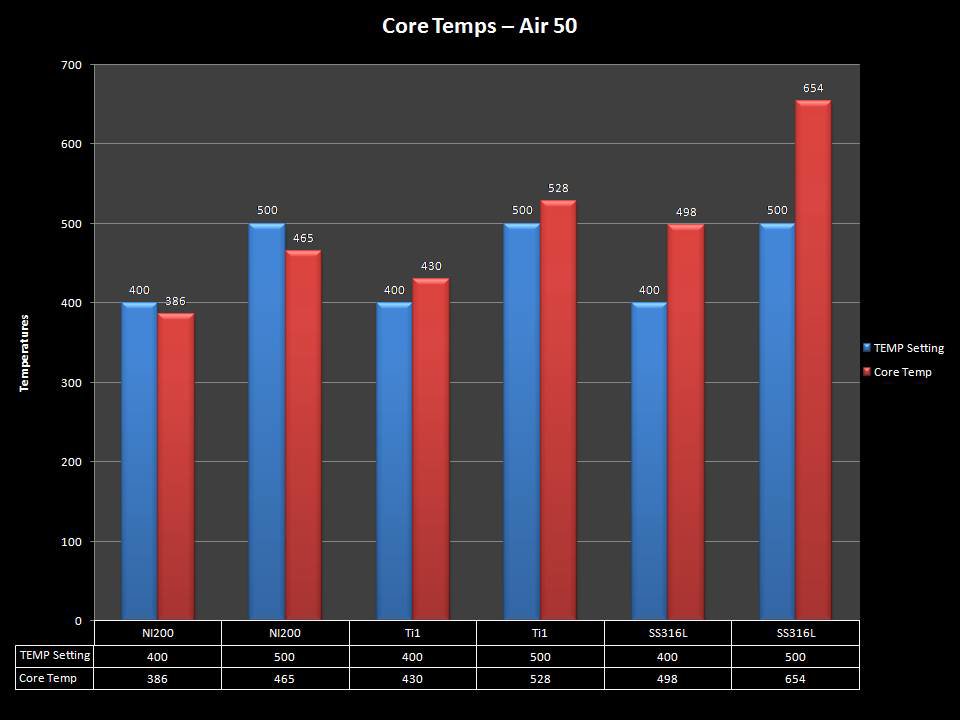
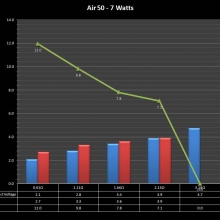
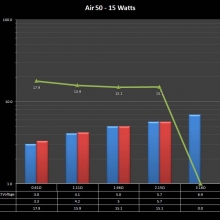
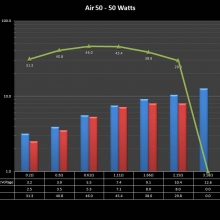
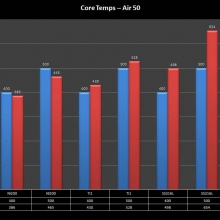
THE “MINI MARATHON” CONTINUES WITH THE SMOKJOY AIR 50 KIT
A PBusardo Review – The Mini Marathon Part 4 – The Smokjoy Air 50 Kit
In this episode of the “Mini Marathon” we take a look at the Smokjoy Air 50 Kit.
The Video:
*NOTE: Any use of these videos in part or in their entirety without Phil Busardo’s expressed written consent is strictly prohibited.
The Photos:
THE “MINI MARATHON” CONTINUES WITH THE ARTERY NUGGET & NEW CONTEST!
A PBusardo Review – The Mini Marathon Part 3 – The Artery Nugget
In this episode of the “Mini Marathon” we take a look at the Artery Nugget and then give one away!
The Links:
Madvapes
Artery Vapor
The Video:
*NOTE: Any use of these videos in part or in their entirety without Phil Busardo’s expressed written consent is strictly prohibited.
The Photos:
A BATTERY MOOCH POST: One Hundred 18650’s tested!
The ratings table has been updated several times the past couple of weeks so if you haven’t downloaded it recently, here it is. Three new Efests, two ESYBs, the Busbars, a Tesiyi, and the VTC5A battery have been added. This brings the total number of 18650’s tested up to 100 and there are still many, many more out there to get to.
Thank you for your support and for helping to spread the word on exaggerated battery ratings!
SMOKE FREE RADIO REPLAY – 6/21/16 – “Industry vs FDA”
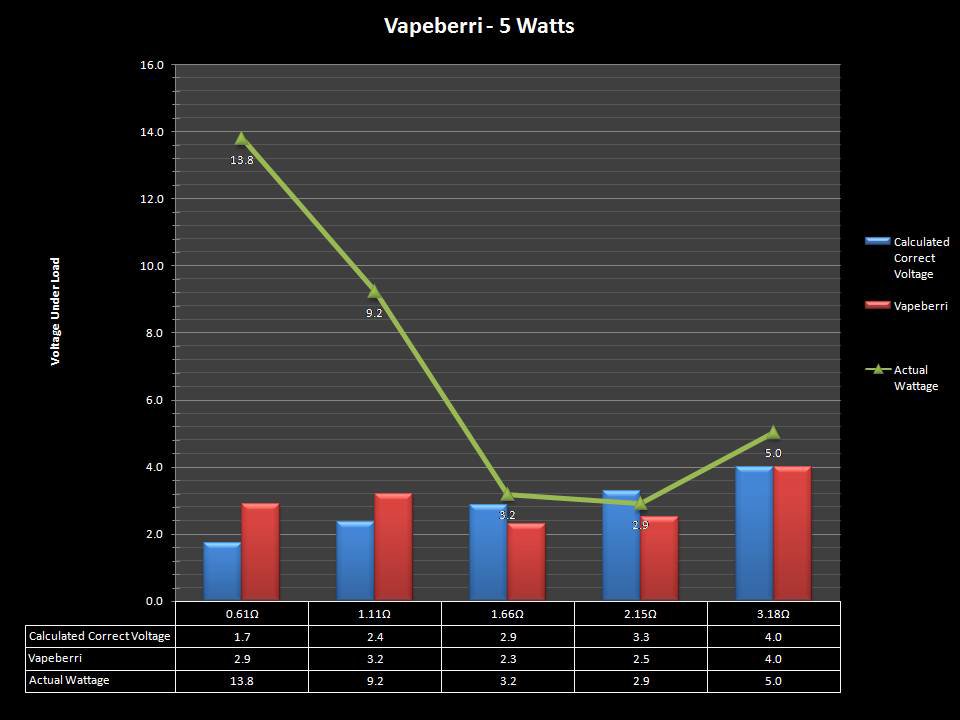
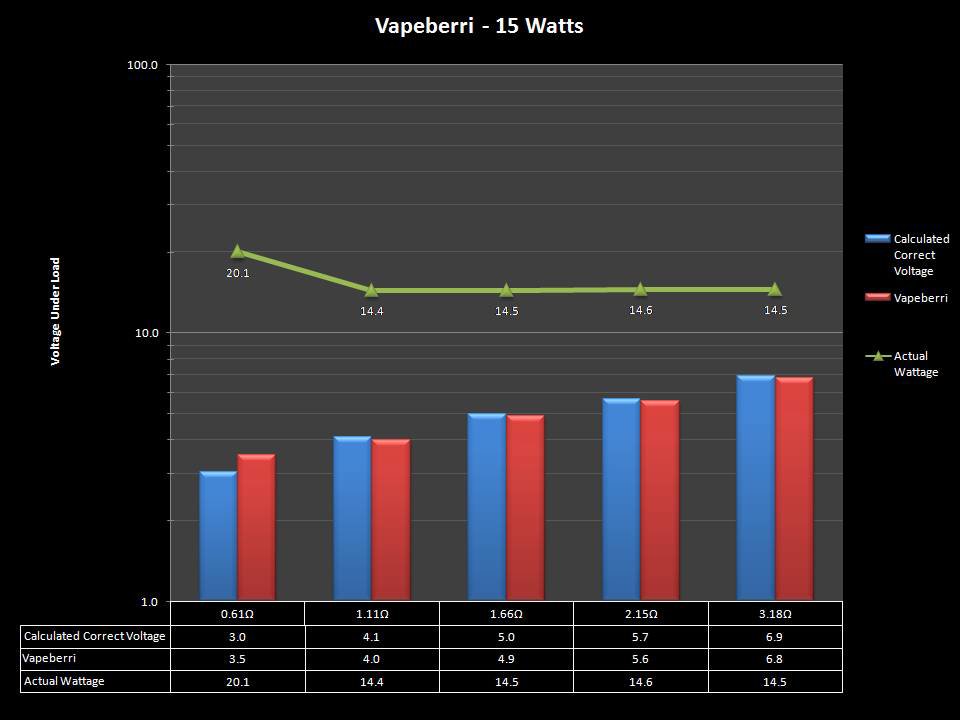
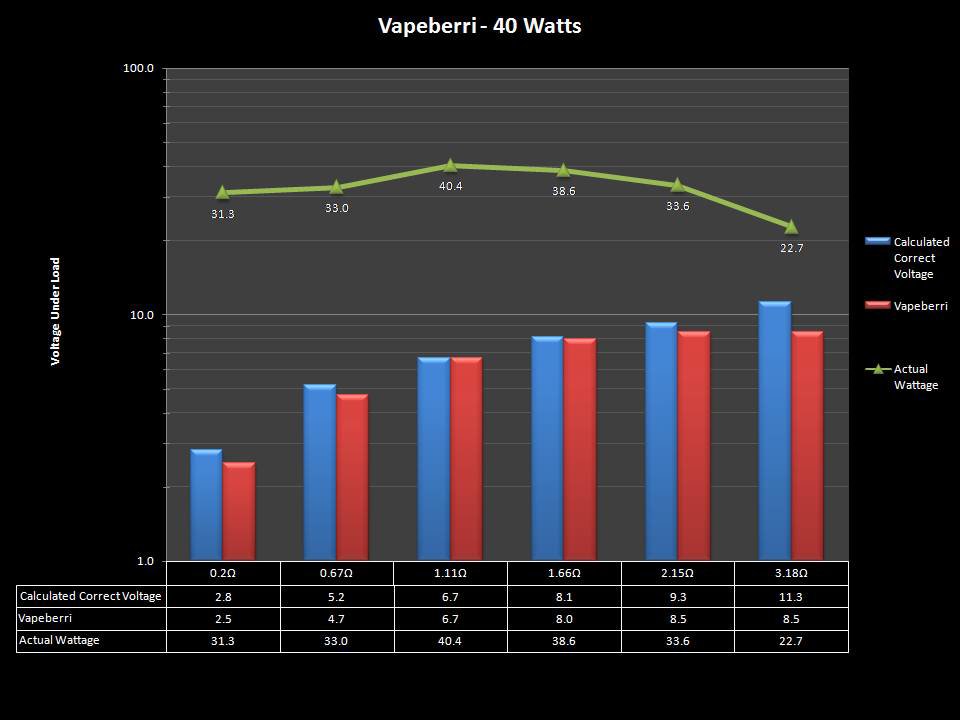
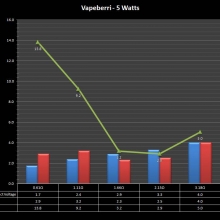
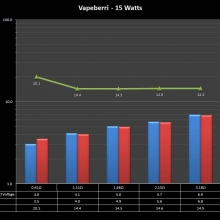
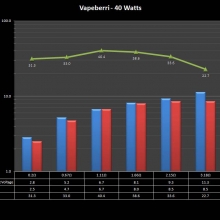
THE “MINI MARATHON” CONTINUES WITH THE VAPEBERRI
A PBusardo Review – The Mini Marathon Part 2 – The Vapeberri
In this episode of the “Mini Marathon” we take a look at the Vapeberri.
The Links:
Eight Vape
The Video:
*NOTE: Any use of these videos in part or in their entirety without Phil Busardo’s expressed written consent is strictly prohibited.
The Photos:
YAY! I GOT MY KAYFUN BACK! YAY!
The MTL reducer kit for the Kayfun V5 came in today and I quickly installed one to see if I’d get my Kayfun back. I did!
The kit comes with 3 pieces which reduces the air channel to 1.2mm, 1.4mm, or 1.6mm.
I selected the 1.6mm, installed it (just presses into the air channel), rebuilt it, and I now have the Kayfun experience I personally look for back. That nice tight, defined throat hit that I look for in my vape, and a draw that is now tight enough for me.
The kit is approximately $10 but I still wished it came with the tank, or was offered for free for those who would like one (Flo shakes his head and curses at me here. 🙂 )
After using the Kabuki for so long, I think I need to compare these two side by side for flavor at some point.
For those who were a fan of the K4 “bulge”, there’s also a new SS tank that gives some of that bulge back with a bonus of added liquid capacity estimated at 7ml, but I haven’t confirmed that yet. This tank is incredibly light, so much so that I thought it was Titanium at first. The walls are only .5mm thick and it weighs in at 9.3g. The tank should cost around $12.50.
Check out some photos below.
SEVIA USA Contributes to the Industry Lawsuit
On behalf of the E-Vapor Industry, Keller and Heckman filed a Complaint in the U.S. District Court for the District of Columbia challenging portions of FDA’s Deeming Regulation and the Tobacco Control Act on various constitutional and administrative grounds. The named Plaintiffs in the lawsuit are:
1. Right To Be Smoke Free Coalition
2. American E-Liquid Manufacturing Standards Association
3. American Vaping Association, Hoboken, New Jersey
4. Electronic Vaping Coalition of America, New Berlin, Wisconsin
5. Georgia Smoke Free Association, Fort Oglethorpe, Georgia
6. Kentucky Vaping Retailers Association, Inc., d/b/a Kentucky Smoke Free Association, Louisville, Kentucky
7. Louisiana Vaping Association, Chalmette, Louisiana
8. Maryland Vape Professionals, LLC, Baltimore, Maryland
9. Ohio Vapor Trade Association, Miamisburg, Ohio
10. New Jersey Vapor Retailers Coalition, Roseland, New Jersey (http://www.njvaporcoalition.org/)
11. Tennessee Smoke Free Association
Also supporting the lawsuit are SEVIA USA and CASAA, AVA, SFATA and NBS.
In the Complaint, we argue the following:
(1) Count I – Violation of Administrative Procedure Act – Grandfather Date: FDA had the authority and the statutory duty to establish a new Grandfather Date for e-vapor products (ENDS) or apply its enforcement authority so that some ENDS manufacturers, including e-liquid companies, would have the opportunity to forego the Premarket Tobacco Application (PMTA) pathway and avail themselves of the option to submit Substantial Equivalence (SE) Reports. By not doing so, FDA violated the Administrative Procedures Act.
(2) Count II – Violation of Administrative Procedure Act – Pre-market Authorization Process: FDA was obligated to consider the continuum of risk of tobacco products and exercise flexible enforcement authority mandated by Congress, instead of a “one-size-fits-all” regulatory regime treating ENDS the same as cigarettes and other harmful products and forcing ENDS manufacturers into the PMTA process, which will all but ban the entire e-liquid and device categories. Accordingly, FDA’s application of the PMTA process to ENDS products violates the Administrative Procedures Act.
(3) Count III – Violation of Due Process and Equal Protection Clauses – Tobacco Control Act: In the Tobacco Control Act, Congress made clear that different tobacco products present different risks and that FDA should exercise its enforcement authority in a flexible manner. But if, as FDA argues, the Agency is mandated to enforce a “one-size-fits-all” regime to all products, including less harmful ENDS, than Congress did not provide FDA with the necessary tools and regulatory flexibility to achieve the Tobacco Control Act’s stated goals, which include allowing newer and safer products to enter the market. As a result, the Tobacco Control Act is unconstitutional under the Due Process and Equal Protection Clauses.
(4) Count IV – Violation of First Amendment and Administrative Procedure Act – Ban on Free Samples: FDA does not have a substantial interest in prohibiting access to free samples of ENDS products by – including taste testing e-liquids in vape shops. The complete ban on free samples does not directly advance the government’s interests. There were more narrow options available to FDA to advance their stated interest in preventing youth access while still allowing vape shops and others to market using free samples. Accordingly, the total ban on free samples violates the First Amendment and the Administrative Procedures Act.
(5) Count V – Violation of First Amendment and Administrative Procedure Act – Modified Risk Tobacco Products: The Modified Risk Tobacco Product (MRTP) provision of the Tobacco Control Act, as applied to ENDS products – which do not produce smoke, combust e-liquid when used as intended, or produce aerosol that contains the harmful substances found in tobacco smoke – does not advance any purported government interests (which focus on traditional tobacco products), and captures commercial and non-commercial speech that is clearly not misleading (e.g., smoke free claims). By applying the MRTP provision and its extensive review process to ENDS products, FDA violated the Administrative Procedures Act and the prior restraint doctrine.
(6) Count VI – Violation of Administrative Procedure Act – Definition of “Tobacco Product” and Application to ENDS: FDA considers a broad range of ENDS products to be regulated as tobacco products or “components or parts,” including software that operates devices, batteries, displays, tanks, etc. FDA intends to regulate these products as tobacco products despite the fact that they do not contain tobacco, are not derived from tobacco and are not components and parts of an actual tobacco product. There is nothing in the legislative history of the Act, and FDA has provided no supporting rationale, for why these items should be regulated as tobacco products merely because they are used to consume the product. Accordingly, FDA’s application of the Tobacco Control Act’s definition of “tobacco product” to certain ENDS is unreasonable and unlawful under the Administrative Procedures Act.
(7) Count VII – Violation of Regulatory Flexibility Act – Unlawful Cost/Benefit Analysis: The Regulatory Flexibility Act requires administrative agencies to consider the effects of their regulatory actions on small business entities. FDA failed to consider significant alternatives, including, but not limited to, the impact of any compliance period on the ability of small entities to successfully navigate the PMTA process given that FDA concedes that there are no long-term clinical studies or other data necessary to support such applications. FDA only considered several, modest alternatives focused on discrete issues like labeling burdens. The Agency did not make a reasonable, good faith effort to consider alternatives that would have an overall impact on all small entities. In short, FDA substantially overestimates the benefits of the Deeming Rule and underestimates the costs. Accordingly, the Court should take corrective action, set aside and remand the Deeming Rule to FDA, and defer enforcement until the Agency complies with the Regulatory Flexibility Act.
(8) Count VIII – Violation of Administrative Procedure Act – Unlawful Cost/Benefit Analysis: The Tobacco Control Act makes clear that FDA was required to adequately consider the costs and benefits of the Deeming Rule. As with the Regulatory Flexibility Act, FDA failed to consider regulatory alternatives, such as an extended compliance period, that would have significantly increased the chance that ENDS manufacturers would be able to comply with the PMTA process, thus avoiding what will be close to an effective ban on ENDS products. The Agency also failed to properly estimate key factors necessary to an adequate cost/benefit analysis, including the number of entities and products affected, as well as the number of PMTA applications that will be filed. For many of these numbers, FDA did not adequately explain or support its conclusions. As a result, FDA violated the Administrative Procedures Act and, therefore, the Deeming Rule must be remanded to the Agency so that a proper cost/benefit analysis may be conducted.
Full complaint can be found here: http://www.khlaw.com/Files/27053_FDA_Deeming_Rule_Complaint.pdf
Dimitris and I would like to thank everyone involved, especially the SEVIA USA founding members which were instrumental in getting this lawsuit off the ground.
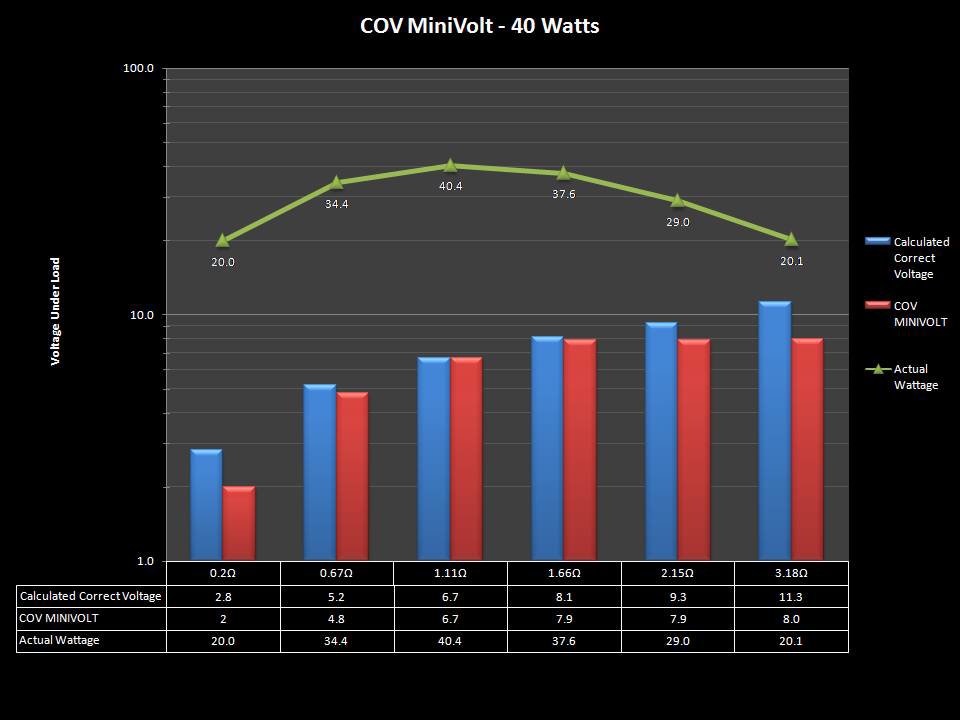
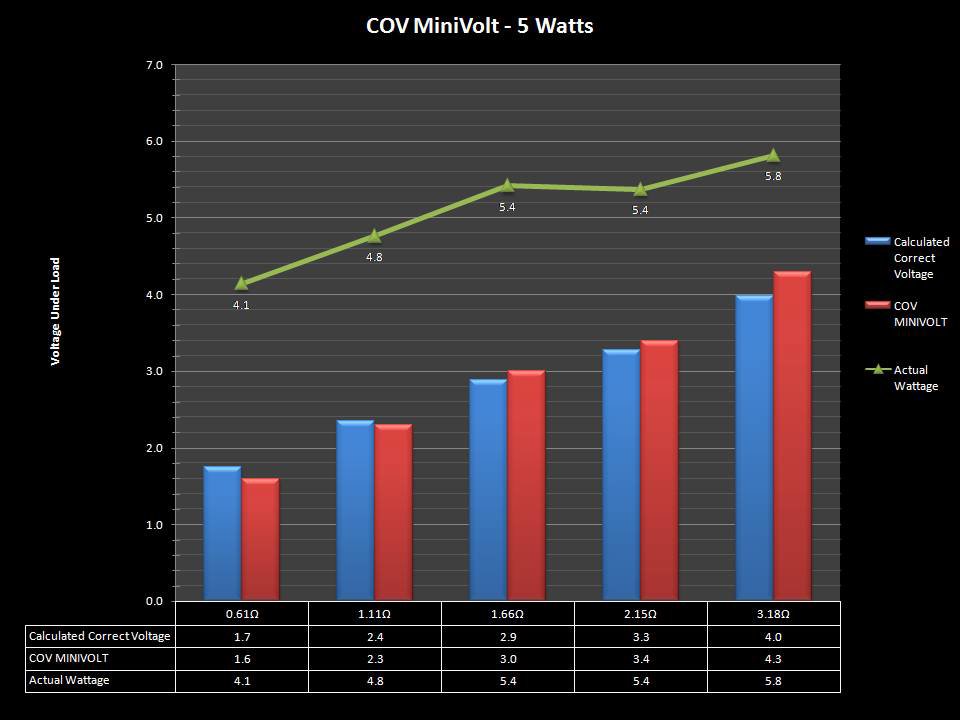
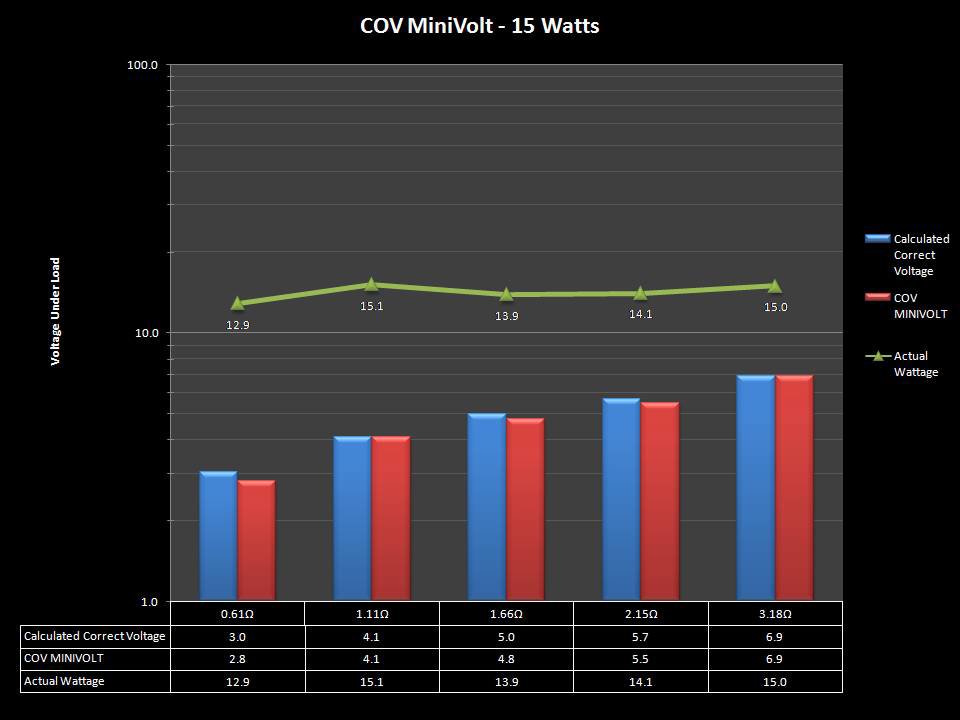
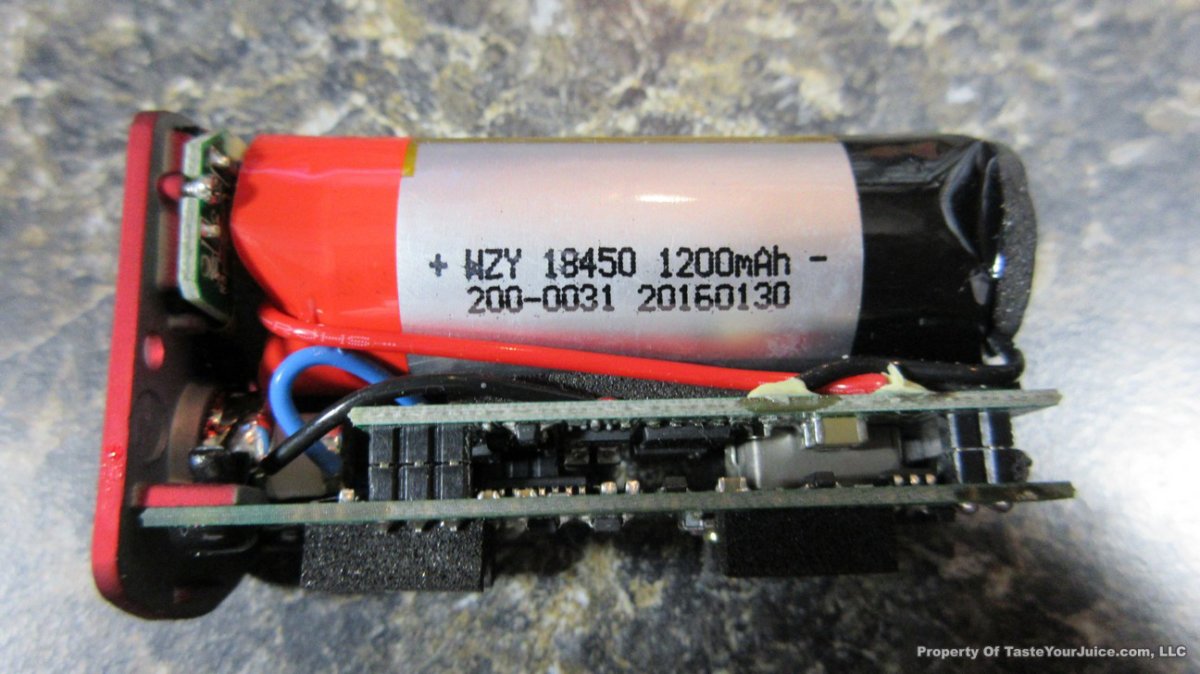
THE “MINI MARATHON” BEGINS… FIRST UP, THE COV MINIVOLT & LAST CONTEST WINNER(S)!!
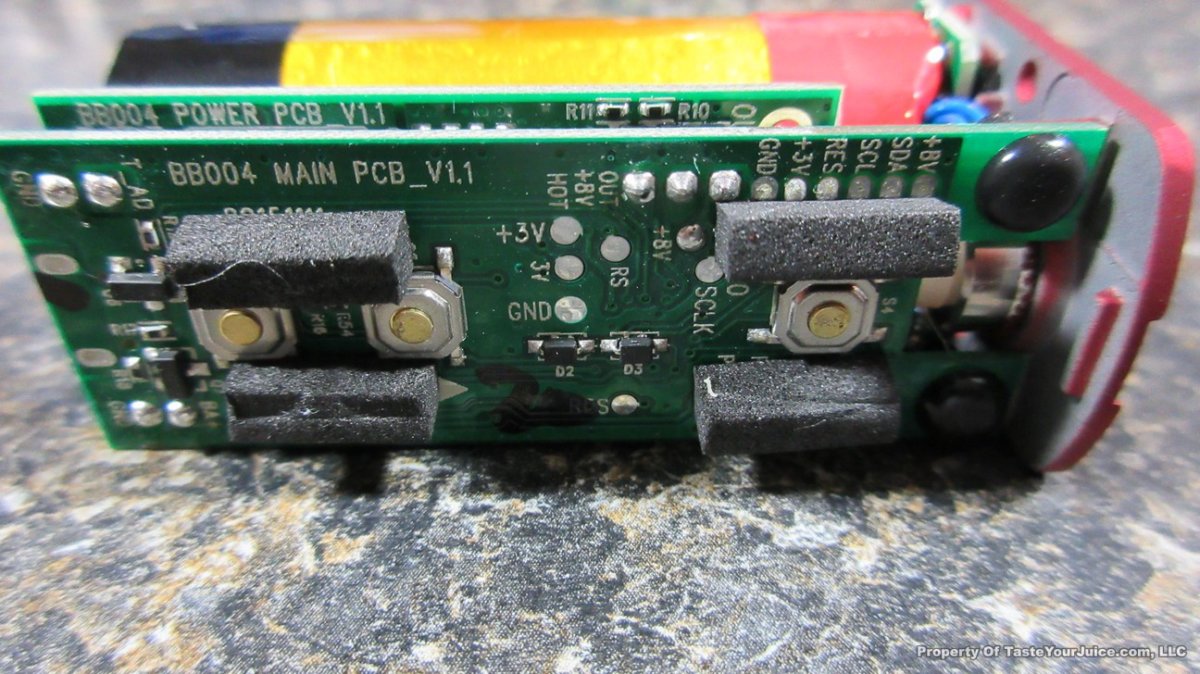
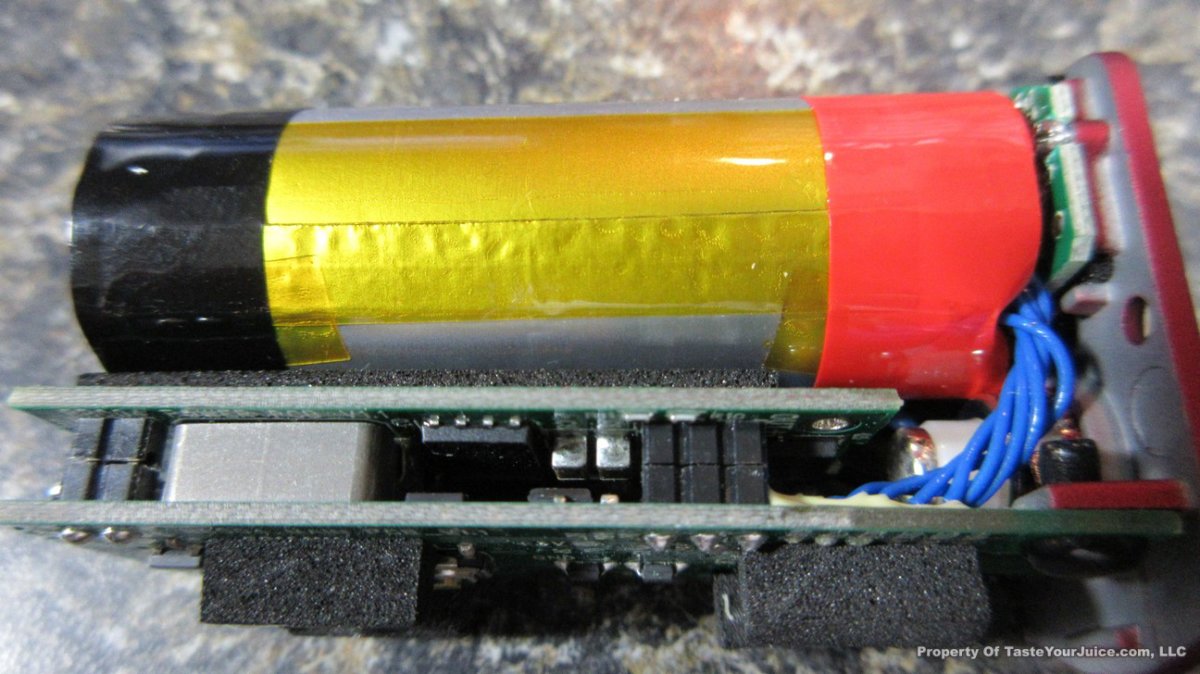
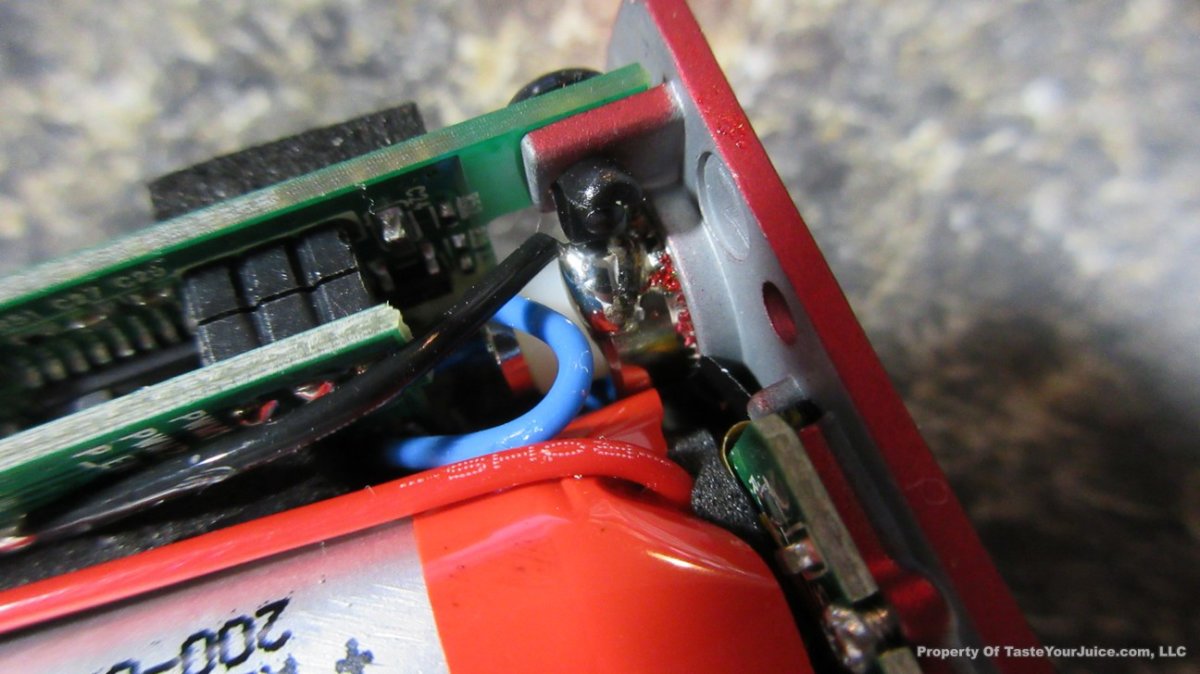
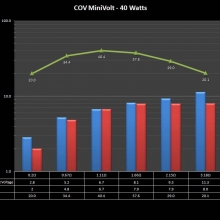
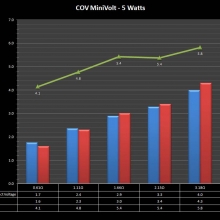
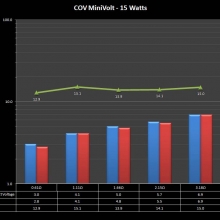
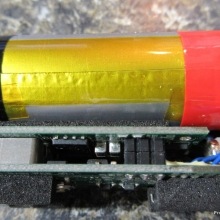
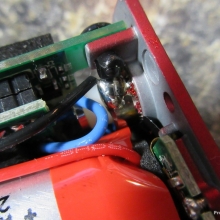
A PBusardo Review – The Mini Marathon Part 1 – The COV Minivolt
We kick off the “Mini Marathon” with this video. A look at some of the miniature devices coming out. Part 1 is a (not so complete) review of the COV Minivolt.
At the end we find out who won the last contest.
The Links:
The Council Of Vapor (COV)
Steeping Giant
Aspire
Kanger
The Video:
*NOTE: Any use of these videos in part or in their entirety without Phil Busardo’s expressed written consent is strictly prohibited.
The Photos:





















































































































































































































































































































































































































































 Store
Store












