Category: Recent News
NEW FROM REGULATOR WATCH – Fabulous GrimmGreen | Vaping Passion, Politics & Art of Regulation
Here’s the latest from Brent Stafford at Regulator Watch:
In the world of vaping video he is one of the most well known content creators on YouTube. He is the indomitable GrimmGreen. Since 2009, Grimm has brought passion, the most up-to-date product knowledge and a deep commitment to vaping advocacy to his audience—now totaling over 300,00 subscribers and over 50-million video views.
Join us for this special edition of RegWatch and learn how Grimm got started, what he thinks of the current state of vaping advocacy and who he thinks is ‘the enemy’ behind the war on vaping—only on RegWatch by RegulatorWatch.com.
RegulatorWatch.com – August 7, 2017.
A (NOT SO) QUICK LOOK – ANGORABBIT COTTON & ANOTHER “NOT A” CONTEST
A PBusardo Quick Look – Angorabbit Cotton & Another “Not A” Contest
In this video we take a (not so) Quick Look at Angorabbit Cotton.
The Links & Contact Information:
Shenzhen Lion Eco-Technology Co.,Ltd.
Contact Name: Trover
Email:trover@angorabbit.com
Skype:Angorabbit Vape Cotton Supplier
Whatsapp:+8615622341211
Facebook:Angorabbit Trover
Post Video Follow-up
I asked a number of questions to Trover based on the comments I received in YouTube. I’ve posted the questions and his responses here along with photos he send me of the cotton growing environment. I appreciate him taking the time to respond to me.
1) Many people are saying in order to get this burn resistance your cotton
is treated chemically. Is that the case?
You have our samples in your hand, can you smell any chemical odor out? Because if the cotton was chemically treated, when you open the package, you can smell the chemical additives directly, or sometimes they use other scents to mask the smell of chemical additives, this is what the most of cotton supplier always did. I believe you can just smell the cotton scents in our product, as I said, it’s pure and 100% organic cotton.
2) On the back of your packaging you have an FDA logo. Why is that? What
is the purpose and meaning of that label?
The FDA logo is just a certification by USA government, nothing special, we just want vapers to know we are certified, not just a unauthentic brand comes out from some unauthentic somewhere.
3) People are calling it Rayon. Is that the case?
I don’t know what some of vapers are calling this, and I don’t know what that means.
4) People are saying is has asbestos fibers. Is that the case?
Asbestos fibers??? I have no idea why they will say that, do you know what is asbestos fibers? That suppose to be tough like steel ! But you have our samples in your hands and I believe you have do a lot of test in our angorabbbit cotton, why don’t you tell me your feeling? You said it’s soft and fluffy right? Do you really believe if we adopts asbestos fibers, it will still be soft and fluffy like this? We have to say, please stop nonsensical attacking.
I would have to agree, this is cotton and not asbestos fibers or rayon. I base this comment purely on the feel of the material.
5) Can you tell me more about the process that gives it burn resistance?
I am sorry but we can not disclose the detail of process to any one, but we do have more than forty professional technology research and more than two hundred fine-tuning of parameters than other normal cotton, the excellent burn resistance is completely in the promise of health that allow vapors have the best experience with the best healthy vape cotton. I know you don’t believe this like what you said in your review video.
Now now, I did not say I don’t believe you, I just said I personally can’t make health claims for the cotton.
So I have to tell you more thing about cotton. All cotton in selling on the market are cosmetic cotton grade, that’s why it can not bear the burning test. But our cotton is specially developed for vaping, only vape cotton need the characteristic of burning resistance, that’s why we took seven months of research and development, finally we got what we want and this is our burning resistance. That’s why some vapers are doubting our cotton of burning resistance.
Angorabbit Growing Environment:
The Video:
*NOTE: Any use of these videos in part or in their entirety without Phil Busardo’s expressed written consent is strictly prohibited.
The Photos:
THE ARTERY LADY Q & A NEW “NOT A” CONTEST!
A PBusardo Review – The Artery Lady Q & New “Not A” Contest
In this video we take a look at the Artery Lady Q. A discreet device designed for a lady. Then we give one away!
The Links:
The Video:
*NOTE: Any use of these videos in part or in their entirety without Phil Busardo’s expressed written consent is strictly prohibited.
The Photos:
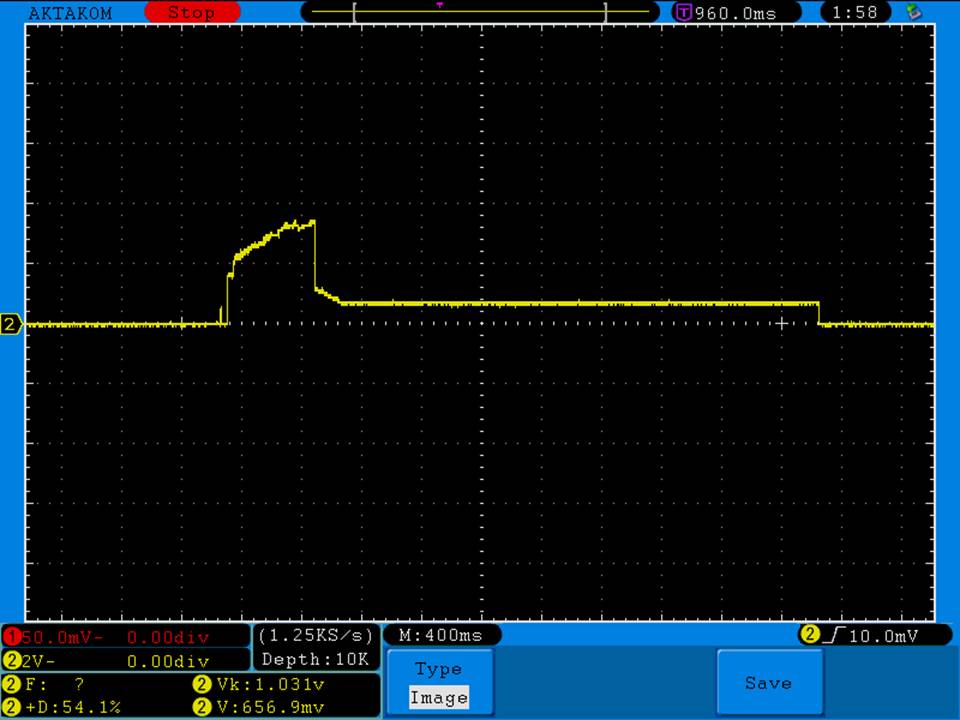
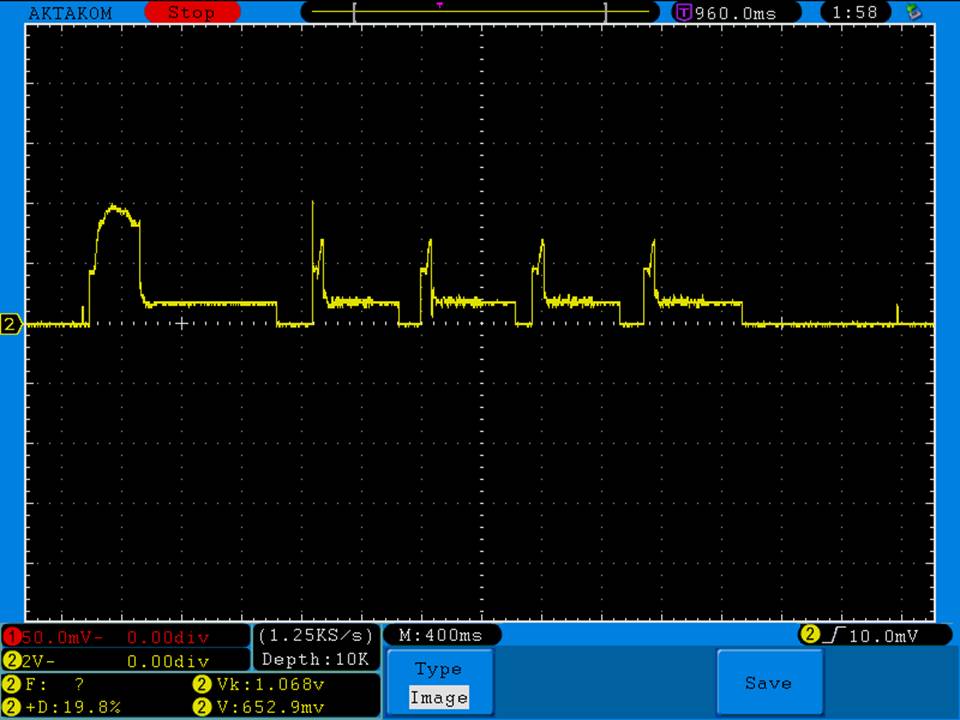
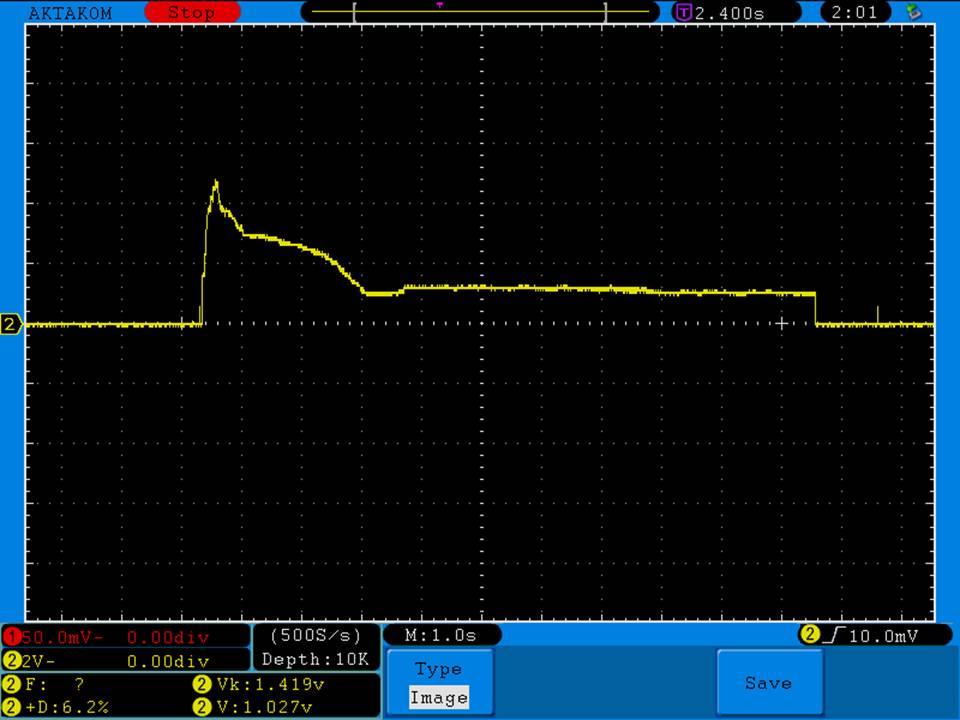
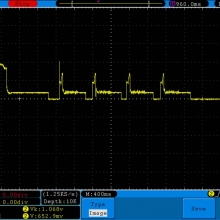
A BATTERY MOOCH POST: Fake LG HE2 alert
My sincere thanks to Danny Carrillo for sending me these fake HE2’s for examination and testing!
Luckily they are terrible fakes and are easily identified, having a three “leg” top contact rather than the correct “four” leg top contact that all LG batteries have.
I want to restate that before all the questions come in…
– This fake HE2 has three “legs” coming down from the top contact.
– The genuine HE2, and all LG batteries we use, have four “legs” coming down from the top contact.
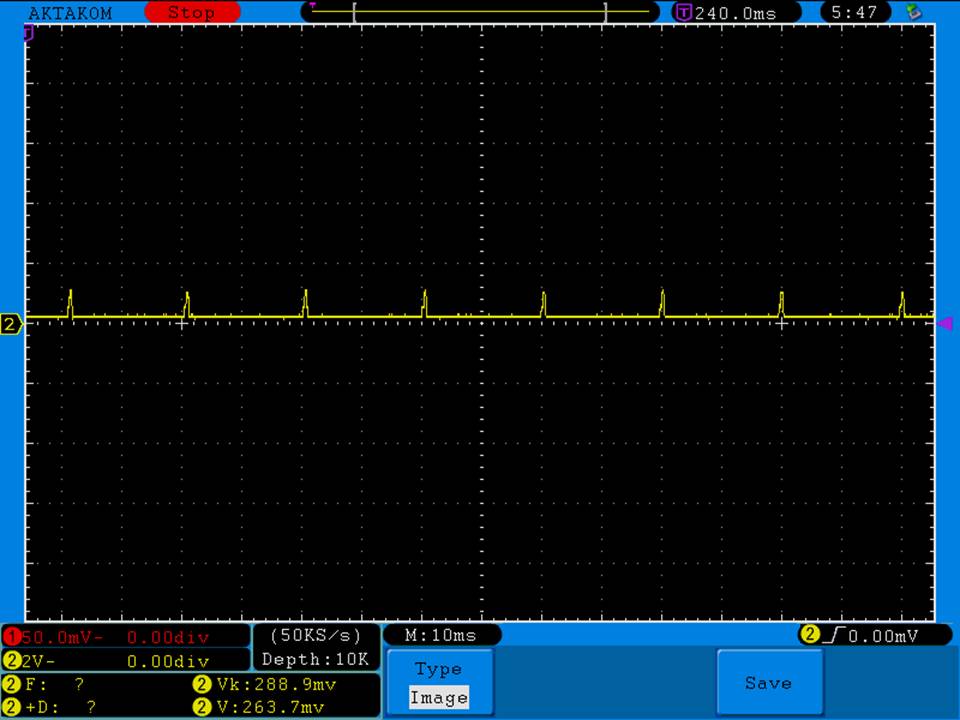
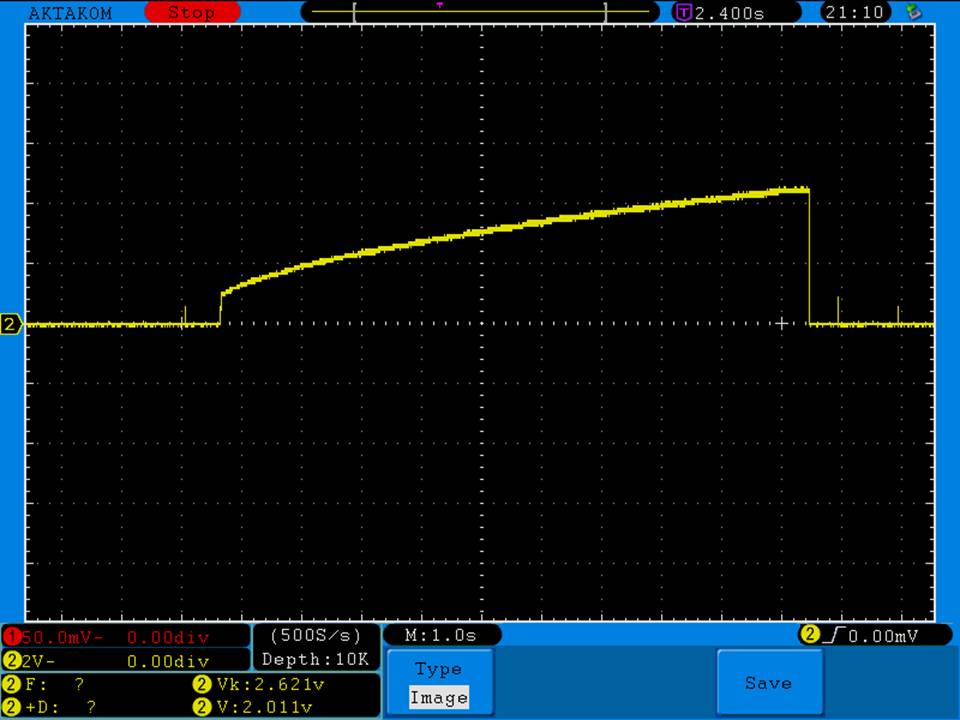
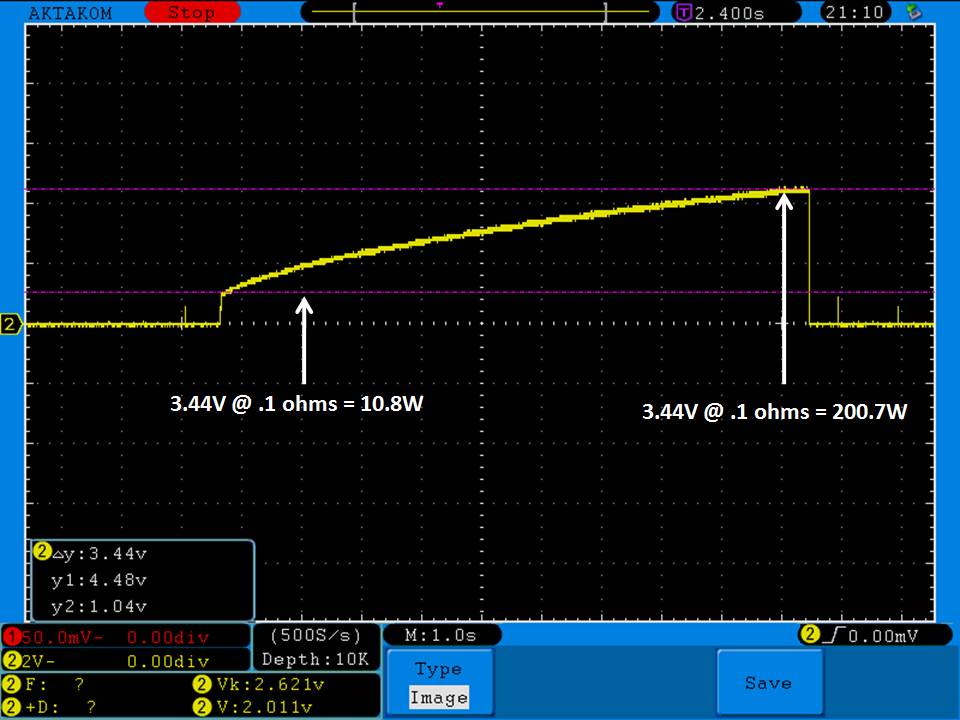
Photos and discharge graphs: https://imgur.com/a/nhPSC
The fakes also have a faint laser etched Greek phi symbol on the bottom. There is nothing on the bottom of the genuine HE2.
The genuine HE2 is a 20A 2500mAh battery. This fake is a lousy performing 2100-2200mAh battery with a 10A-15A rating. But, there might be more than one type of battery being rewrapped for these fakes. I can only talk about the ones I have.
I don’t know what battery is being used for these fakes.
If you have any of these fakes then contact the vendor you purchased them from to see if you can get a refund. If you insist on using them then carefully treat them as crappy 10A batteries. I recommend just returning or recycling them though.
PLEASE READ:
– The date/batch code on the wrap of the fake might be a genuine code. This alert is NOT saying that all HE2’s with that code are fake!
– LET ME REPEAT THAT: THIS POST IS NOT SAYING ALL HE2’s WITH THAT CODE ARE FAKES! Only that these fakes are using that code.
– This alert is NOT saying that all HE2’s with a four “leg” top contact are genuine! There might be other fakes out there using genuine LG batteries with lower current ratings.
– I am NOT saying anything other what I am saying directly in this post.
– Please do not send me pictures of your HE2’s. I am unable to authenticate your batteries via photos. Use the pictures here to determine if yours are fakes or not. If you are still unsure then CAREFULLY compare the performance of the suspect batteries against known genuine HE2’s, HE4’s, or 25R’s (their performances are all about the same). If you are still unsure, return or recycle them.
Thanks!
Mooch
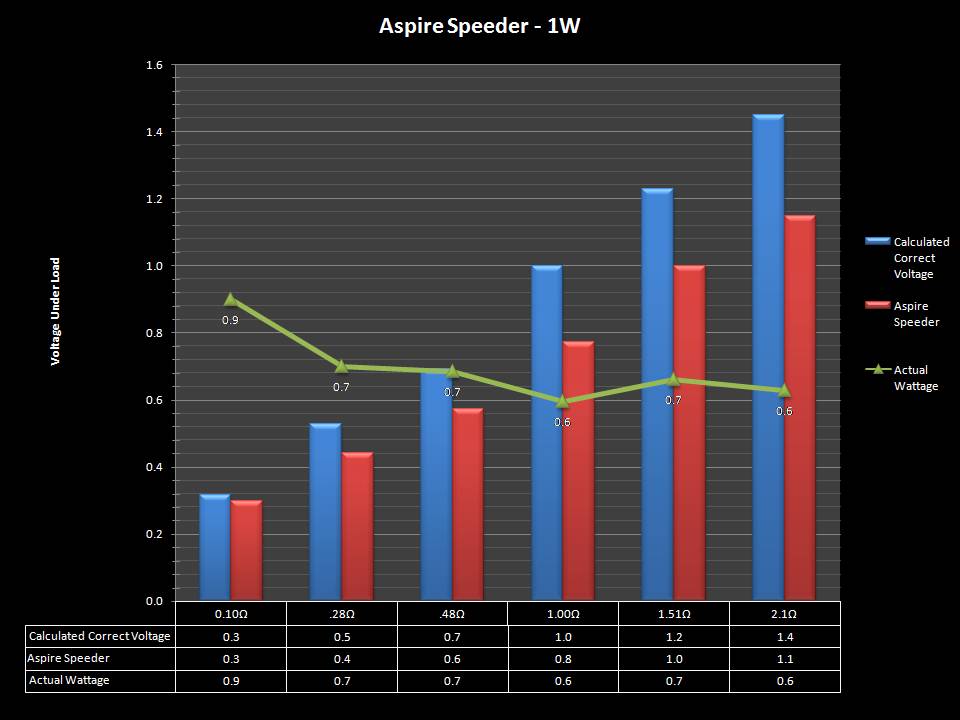
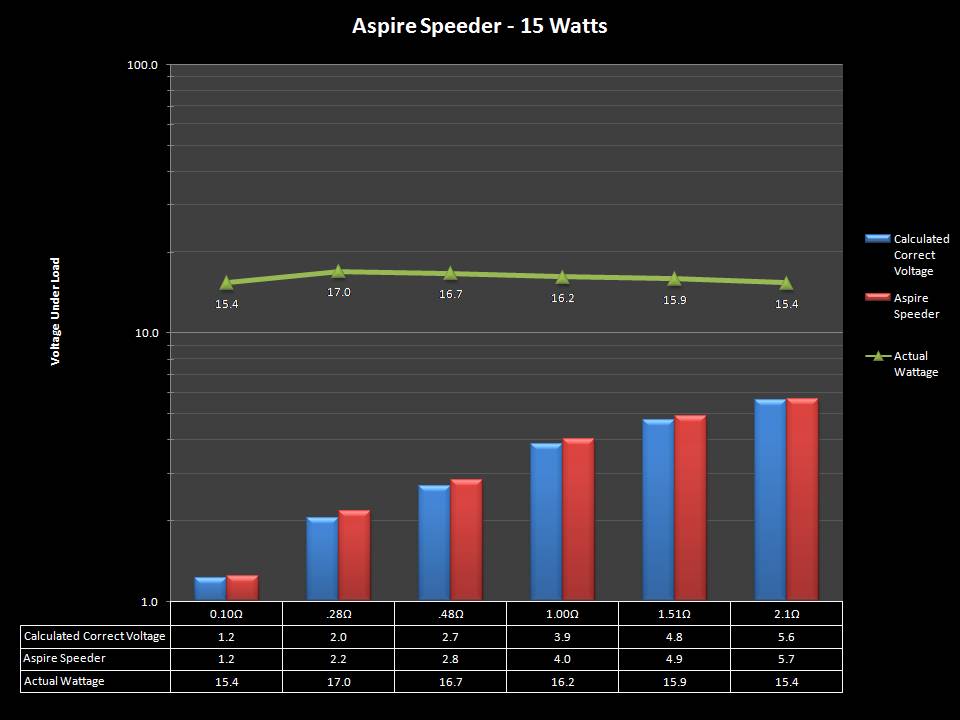
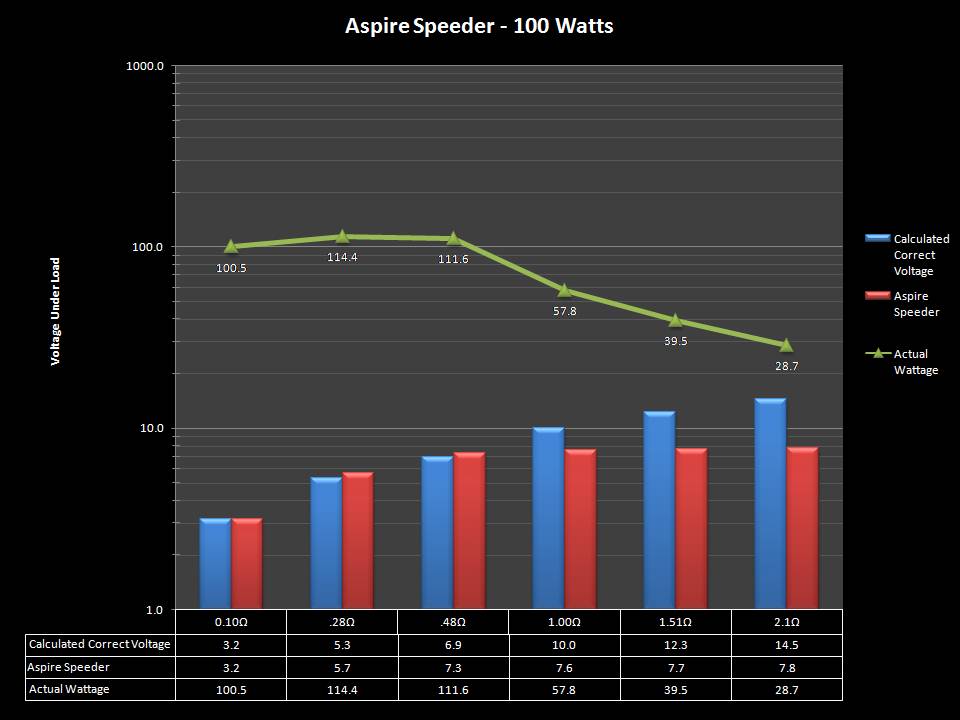
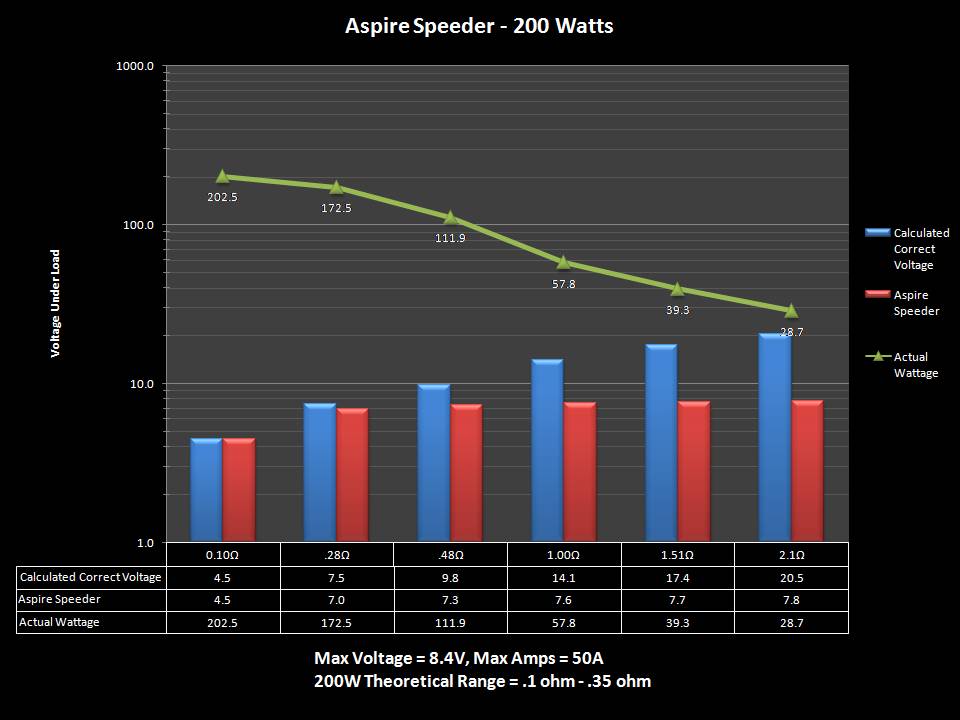
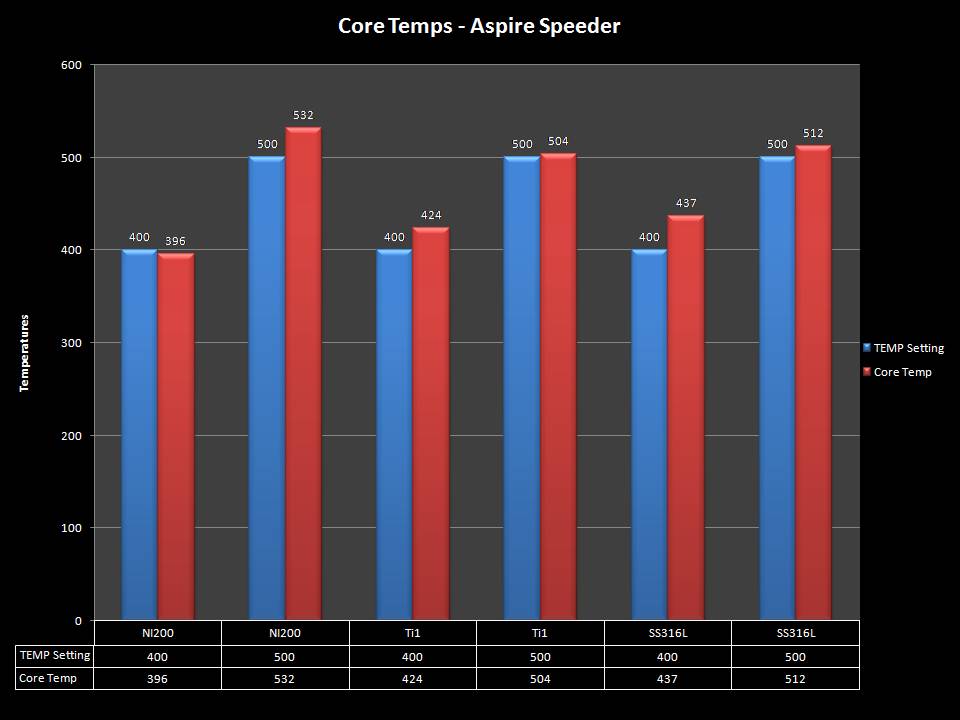
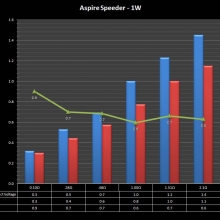
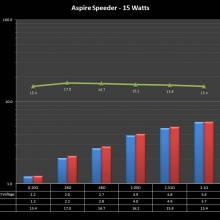
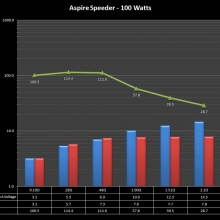
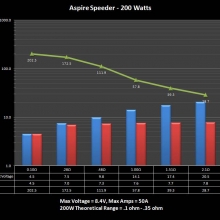
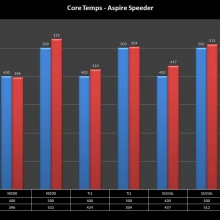
THE ASPIRE SPEEDER KIT
A PBusardo Review – The Aspire Speeder Kit
In this video we take a look at the Aspire Speeder Kit.
The Links:
The Video:
*NOTE: Any use of these videos in part or in their entirety without Phil Busardo’s expressed written consent is strictly prohibited.
The Photos:
NEW FROM REGULATOR WATCH – Ground War | Regulatory Insights on Bill S-5
Here’s the latest from Brent Stafford at Regulator Watch:
A golden opportunity, that’s how regulatory lawyer Rajeev Sharma describes the landscape facing the vaping industry as Bill S-5, Canada’s draft federal vaping regulations, continues its march through Parliament.
What steps should the vaping industry and advocates take to win over the hearts and minds of Canadian political leaders? Is Health Canada hedging its bets? And, is it time for a ground war in support of vaping?
Get inside insights from the leading regulatory advisor to the Canadian vaping industry—only on RegWatch by RegulatorWatch.com.
RegulatorWatch.com – August 3, 2017.
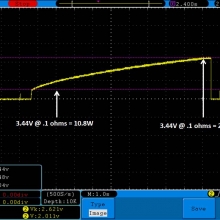
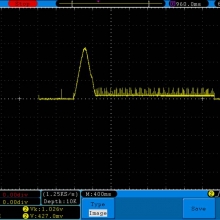
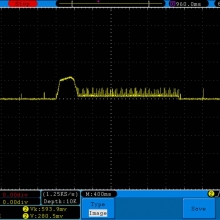
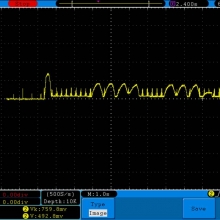
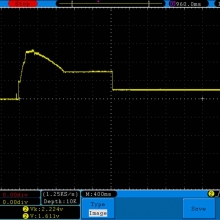
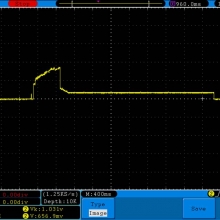
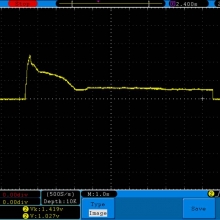
A BATTERY MOOCH POST: Golisi 35A 4300mAh 26650…a great 31A battery, close to the iJoy
Bottom Line: For the GeekVape Aegis mods that ship with a battery, this is it. This a great performing cell, easily one of the top three 26650’s. It is equal to the Aspire 4300mAh (test results soon) and almost equal to the iJoy 4200mAh. The Golisi is identical in appearance to the Aspire and only the surface finish of the top contact metal differentiates it from the iJoy.
I will be retesting the iJoy again soon to see if these three might all be the same cell.
This cell runs a couple of degrees cooler than the iJoy so it earns a 31A continuous rating versus the 30A rating of the iJoy. You will not notice a difference this small in use though. The iJoys I tested in September, 2016 hit a little bit harder though.
I am rating this Golisi cell at 31A and 4300mAh. I’m not bothered too much by Golisi’s 35A rating because it’s so close to mine.
The two cells that were tested were donated for testing by Geekvape (www.geekvape.com). Thank you!
Test results, discharge graph, photos: https://www.e-cigarette-forum.com/forum/threads/bench-test-results-golisi-35a-4300mah-26650-a-great-31a-battery-close-to-the-ijoy.819859/#post-19864007
All my test results to date: https://www.e-cigarette-forum.com/forum/blog-entry/list-of-battery-tests.7436/
FROM TONY ABBOUD / VTA – FROM THE TRENCHES: VTA UPDATE

FROM THE TRENCHES: VTA UPDATE
August 2, 2017

THE FEDERAL FRONT
LETTER FROM VTA’s BOARD OF DIRECTORS
Since January 2016, VTA has charted a course for the vapor industry by putting together a professional team that could execute a comprehensive strategy at the federal and state levels. First, we implemented a two-phase strategy at the federal level. Phase 1 was designed to keep our vibrant and diversified industry alive by changing the predicate date via legislative initiatives and delaying the implementation of the Deeming Regulation deadlines via executive initiatives. Phase 2 was designed to secure the long-term future of our industry through establishing a rational set of regulations. To facilitate the short-term and long-term game, we have been focused on delivering the appropriate messages to decision-makers in Washington, D.C. regarding the unprecedented public health benefit of vapor products and the enormously significant job-creating, small-business component of our industry.
This month marks another major milestone in our efforts.
On July 12, the House Appropriations Committee included in its base bill, and then passed, the Cole-Bishop language that would change the predicate date for vapor products. As such, we have for the second time passed pro-vapor legislation out of the House Appropriations Committee.
On July 28, FDA Commissioner Gottlieb made a major policy speech in which he granted one of the main points of relief we have been seeking – a four-year extension of the PMTA deadlines.
As many of you know, these successes required us to organize on-the-ground efforts in D.C. and deploy resources in many states through numerous channels. We are incredibly pleased with these recent events, and are grateful for all those who worked with us, but you need to know that VTA is just getting started. There is still an enormous amount of work to be done to restore innovation to the U.S. marketplace as we move on to our Phase 2 – establishing a rational set of regulations that recognize and endorse the life-altering benefits of vapor products. If you like any of what we have been doing and if you are enjoying the benefits of our efforts, then we are calling on you – all vape shops and all vapor companies seeking to secure our industry’s future – to join our strategic, professional and effective efforts.
George Cassels-Smith, e-LiquiTech
Seth Coblenz, VMR Products
Brittani Cushman, Turning Point Brands/Vapor Beast
Arnaud Dumas de Rauly, GaiaTrend
Stacey Hamilton, Kaleidoscope Vapor Lounge
Chris Howard, E-Alternative Solutions
Patricia Kovacevic, Nicopure Labs
Ron Tully, Next Generation Labs
VTA LEADS THE WAY TO A MAJOR VAPOR MILESTONES IN D.C. AS FDA ANNOUNCES 4-YEAR DELAY IN PMTA DEADLINE
In what can only be considered a sea change in the way that FDA is examining vapor products, last week VTA realized two of its principal governmental objectives when FDA Commissioner Scott Gottlieb made his major announcement on how FDA would view and treat vapor products going forward. Most significantly, Commissioner Gottlieb, flanked by Center for Tobacco Products Director Mitch Zeller, announced the FDA’s “Protecting American Families: Comprehensive Approach to Nicotine and Tobacco.” Commissioner Gottlieb announced that FDA is implementing regulatory changes to “ensure that the FDA has the proper scientific and regulatory foundation to efficiently and effectively implement” the Tobacco Control Act and, in doing so, granted a four-year extension of the deadline for Premarket Tobacco Applications for Electronic Nicotine Delivery Systems (ENDS) products until August 8, 2022.

The FDA’s policy shift is described in Commissioner Gottlieb’s full remarks and follows intensive efforts by VTA and others to convince the Administration that something dramatic needed to be done to fully recognize the enormous public health potential of vapor products. Since we launched, VTA, along with public health and other vapor advocates, has been delivering the message that our government needs to embrace and promote the promise of vapor products which, according to the most recent NIH-funded research out of the University of California published in the British Medical Journal, are very effective tools in helping addicted smokers quit.
VTA wanted to take advantage of the fact that our national industry trade association was uniquely positioned to secure and conduct meetings with key decision-makers within the Administration. So, immediately after the inauguration, VTA tasked its governmental affairs team to educate directly HHS and the White House.
On April 24, 2017, VTA’s Board and leadership team met with the key decision makers at the Department of Health and Human Services (HHS). Specifically, VTA met with senior leadership at HHS and presented a strong and detailed case for why vape shops and manufacturers all across the country need relief from the current Deeming Regulation. In addition, we presented some of the significant positive scientific research that was not considered by FDA during the comment period of the Deeming. Also, we took the opportunity to personally deliver to HHS senior staff the letter to Health and Human Services Secretary Tom Price, which was signed by over 2,000 vapor business owners from all 50 states urging the agency to take immediate action to dramatically delay the FDA’s May 2016 Final “Deeming” Rule.
Then, on May 5, 2017, in a first-of-its-kind meeting, VTA’s Board and leadership team took the vapor industry’s case directly to the White House. VTA met with senior leadership of the Domestic Policy Council (DPC), the Administration’s health care policy leadership, as well as DPC staff. As previously reported, DPC leadership was fully engaged in our discussion and very interested in both the negative health and economic implications of the Deeming Regulation. We were impressed by their knowledge of our industry which enabled us to get into the details of why the current Deeming poses such a threat to our industry, why small businesses are being forced to close down, and why manufacturers simply cannot be asked to comply with PMTA requirements.
Most importantly, we were able to explain why the FDA’s then recently-announced 3-month delay (connected to the ongoing litigation) and any continued short-term delays would not be helpful to the industry. We presented information to demonstrate that short term-delays were unworkable for large businesses trying to decide on whether to spend millions on PMTA compliance and irrelevant to small businesses trying to decide whether to sign a multi-year lease.
Finally, we emphasized the importance of implementing a long-term, minimum two-year, delay so that we could quickly turn to implementing a regulatory scheme that properly recognizes the uniqueness of vapor products and the remarkably important role they play in harm reduction. Then, to bolster that message and address specific questions raised by the decision-makers, VTA drafted a legal, factual, and policy brief justifying a major delay of the Deeming Regulation deadlines and provided that analysis to both HHS and the White House later in May.

Since then, FDA clearly spent the past few months working on the significant shift in policy announced last Friday. The current state of science demonstrates that ENDS products are at least 95% safer than combustible cigarettes. FDA is taking a big step forward in protecting public health by acknowledging for the first time that ENDS are a harm reduction product and need to be regulated as such. To be sure, FDA’s new approach is a thoughtful response to and recognition of the need for science-based regulation, rather than a knee-jerk response to threats of litigation.
By delaying the PMTA compliance deadlines for ENDS, FDA has also recognized that the current regulations have halted the kind of technological innovation that is key to ending this country’s reliance on combustible cigarettes, and that we need to implement clear and meaningful regulations both to protect consumers and foster innovation. To that end, we are encouraged by this passage in FDA’s Press Releasewhich reflects a clear recognition of the message that VTA consistently presented to the Administration: “This action [delay of PMTA deadlines] will afford the agency time to explore clear and meaningful measures to make tobacco products less toxic, appealing and addictive. For example, the FDA intends to develop product standards to protect against known public health risks such as electronic nicotine delivery systems (ENDS) battery issues and concerns about children’s exposure to liquid nicotine. It also will provide manufacturers additional time to develop higher quality, more complete applications informed by additional guidance from the agency.”
Since May 2016, when the Deeming Regulation was published, VTA has been pushing for a rational set of regulations on ENDS products that would be based on science, rather than fear. In addition, we have been advocating for a pause in the current regulations to allow for a more thoughtful, science-based regulatory policy to take root. The decision by FDA is the right thing for the country and in the best interest of public health, and we commend Commissioner Gottlieb and his staff for putting politics aside and implementing fact-based policies that are in the best interest of the country.
VTA is prepared to work closely with FDA and other stakeholders to ensure that we develop a set of regulations that protect consumers, promote public health and protect the small and mid-sized businesses that are the backbone of the vapor industry.
Notwithstanding these exciting developments, the vaping industry needs to clearly understand that we must be more vigilant now than ever and more steadfast in our determination to re-shape the regulations governing our technologies. For example, the FDA has indicated that it is going to engage in additional rule-making regarding batteries and flavors. In addition, there are other compliance deadlines that were not directly addressed in the Commissioner’s announcement that need to be addressed and VTA already is looking for ways to do so.
VTA’s Board of Directors has incredible insights into and experience with the FDA regulatory process. Moreover, VTA has developed a reputation among elected and Administration officials as a leader in the industry, as the voice representing manufacturers and retailers able to provide clear arguments for practical regulation, and as a resource for data and economic impact of over-regulation on the growing industry. For all these reasons, VTA is uniquely positioned to steer our industry forward and we are inviting you to work with us to secure the future of the vapor industry.
VAPE & THE FDA 2 INDUSTRY CONFERENCE A SUCCESS
Surviving & Thriving in A New Regime for Vapor
On July 18, 2017, VTA hosted its second annual conference – Vape & the FDA 2: Surviving & Thriving in A New Regime for Vapor at the Trump International Hotel in Washington, D.C. More than 150 people, from 23 states came together to listen to some of the top voices and thought leaders in our industry and discuss strategies for the future. We will be publishing a full summary of everything that occurred at the conference, including the new state and local initiatives that were presented by VTA, CASAA, and state vapor association leaders but what follows are some highlights of the conference.

The conference was kicked off by keynote speaker Senator Ron Johnson (R-WI), Chairman U.S. Senate Committee on Homeland Security and Governmental Affairs. Chairman Johnson gave a rousing address to the attendeesstressing the importance of the work that VTA was doing and the need to continue to work on Members of Congress. In addition, Chairman Johnson clarified that the strategy we have been focused on is the right one and, in response to questions from the audience, he was kind enough to explain why other popular approaches (i.e., coordination and stand-alone vapor legislation) were either unrealistic or unworkable. The room was thrilled to be able to give the Chairman a standing ovation for his full-throated defense of small businesses and the vapor industry.

The afternoon was carved into a dual track: one for manufacturers’ compliance with PMTA, and one for retailers and state vapor association growth strategies, before we came back together to hear Dr. Sally Satel give a thoughtful defense of tobacco harm reduction and vapor products. Jake Butcher, VTA State Affairs Manager, gave attendees an incredible summary of the enormous successes that VTA and its affiliated state vapor associations have enjoyed at the state level and led a state taxation panel discussion with state lobbyists and industry professionals fighting against vapor taxes.
New VTA Initiatives Announced:
During the conference, Tony Abboud presented VTA Today and Tomorrow, describing the targeted initiatives that VTA has conducted with senior leadership at the White House and HHS, the strong small business message that we delivered, and the going forward strategy to defend the vapor industry. As part of his presentation, Abboud announced VTA’s initiatives for initiatives for the second half of 2017, including:
(1) expanding VTA’s Board of Directors;
(2) assembling VTA’s Retailer Advisory Council to focus on and address issues unique to retailers;
(3) rolling out VTA’s Strategic Local Defense Initiative to coordinate, plan and defend the vapor industry at the local level through a joint vape shop / consumer strategy planned with CASAA; and
(4) establishing VTA’s industry Standards Task Force to focus on setting the correct base for e-liquid and device regulations for the vapor technology industry.
Immediately following the action-packed conference, attendees got to enjoy a great reception that allowed vape shop owners, manufacturers, distributors, importers, and suppliers to network and relax in comfortable surroundings.
Day on the Hill: 
On July 19, VTA’s lobbying team – West Front Strategies – executed another fantastic Day on Capitol Hill, setting up more than 90 meetings and leading more than 100 vape shop owners, state vapor association leaders,manufacturers, wholesalers, distributors, and consumers to Capitol Hill. Our ask to Members of Congress was to assist us in halting or dramatically delaying the FDA’s May 2016 Final “Deeming” Rule at the risk of shuttering thousands of small vapor businesses across the country. We explained that the rule, which had been hastily implemented, does nothing to address product standards or consumer safety issues, and is so overly burdensome that it is tantamount to a ban on these new and innovative vapor products.
 VTA Members asked for passage of HR 1136 and that Members of Congress send a pre-drafted letter to FDA Commissioner Scott Gottlieb encouraging him to delay by at least two years the continued implementation of the Deeming Regulations. We were thrilled that many Members of Congress agreed to send the letter or reach out to Commissioner Gottlieb right away and that they followed through on that promise!
VTA Members asked for passage of HR 1136 and that Members of Congress send a pre-drafted letter to FDA Commissioner Scott Gottlieb encouraging him to delay by at least two years the continued implementation of the Deeming Regulations. We were thrilled that many Members of Congress agreed to send the letter or reach out to Commissioner Gottlieb right away and that they followed through on that promise!
We will distribute a full report on the conference shortly, but with the big news this week coming shortly on the heels of our latest push in Washington, D.C., we wanted to focus on that issue first.
THE STATE OF THE STATES
 PVA and VTA Are the Vanguard for Pennsylvania Vape Shops
PVA and VTA Are the Vanguard for Pennsylvania Vape Shops
In the past two weeks, the Pennsylvania vape industry’s attempt to unwind the harm created by the business killing 40% wholesale tax was twice challenged. Unfortunately, those challenges were not mounted by anti-vaping groups but from what should be vape-friendly groups.
First, non-business owners attempted to discredit the motives of PVA by claiming this vape shop group was doing the bidding of “big-tobacco” and by demanding that the business-killing 40% tax remain in place. Their bizarre positioning on this issue completely ignored some basic business realities that small business owners, by contrast, do understand. For example, vape shop owners understand that a new point-of-sale per ml tax will (a) keep money in their pockets; (b) improve their cash flow and operations; (c) remove a hurdle for those shop owners looking to re-open their businesses; (d) require new negotiated competitive pricing structures – since that is what in fact occurs in a free market; and (e) tax every milliliter of e-liquid at the same rate, regardless of the form in which it is sold.
Second, on the Senate side, PVA and its lobbying team quashed a surprise attempt by forces to include a $0.15 per ml tax in the Senate tax code bill against Sen. Bartolotta’s wishes. Thanks to quick action by PVA’s Board, and by VTA and the Kinser Group pulling out all stops, we got the $0.15 per ml tax stripped out of the Senate appropriations bill before it was published, even though it was supported by big tobacco.
Make no mistake, PVA and its lobbyists are moving mountains and changing the entire discussion in Harrisburg during one of the most difficult sessions in history. Without their action, the House would currently be negotiating against a $0.15 per ml Senate tax right now. The fact that we are still in play and that we are having this kind of impact this late in the session is a true testament to the Kinser Group’s efforts and the team that PVA has put together. If you are not a member, click here to join and check out PVA’s membership options.
NOW IS THE TIME TO JOIN VTA!
As you can see from the foregoing, VTA is not only set up for success but is the driving force for the vapor industry’s success to date. We have an outstanding Board of Directors comprised of vapor manufacturers and retailers who have deep FDA and industry experience, we have a bi-partisan team of five lobbyists (known as the vapor lobbyists) on Capitol Hill, we have a bi-partisan public affairs team who are expert at messaging, and we have a State Affairs Manager (also a state lobbyist/lawyer) who is in constant touch with state vapor leaders and their lobbyists.
Our members get access to these professionals and lots of meaningful guidance on all the federal and state regulatory and strategic issues affecting our industry. And, if ever you want to get me on the phone, here’s the number: 312-498-6060.
In short, if you like what you read in this update and if you want to be a part of securing our industry’s future, then join us and the hundreds of other companies we are proud to call VTA Members today! All you need to do is fill out this VTA Membership Application and return it to us.
To learn more, check us out at www.vaportechnology.org and www.SaveVapor.org.
And, don’t forget to follow us on Facebook and Twitter.
There is so much work to be done, but VTA is just getting started.

Tony Abboud
Executive Director
Vapor Technology Association
From Doctor Farsalinos – FDA announcement: a policy change of endorsing harm reduction?
In conclusion, this FDA announcement is definitely a positive step, but there should be a follow up. The current regulatory framework is extremely expensive and complex, and will largely create an oligopoly and stifle innovation. Unless changes in the process and requirements of acquiring a license are implemented, the change in the deadline for submissions will just delay the virtual elimination of the e-cigarette market as we know it today. Of course regulation and product standards are needed, but the FDA needs to find the ideal balance in preparing a reasonable, realistic and proportionate regulatory framework.
Read the entire article HERE
.
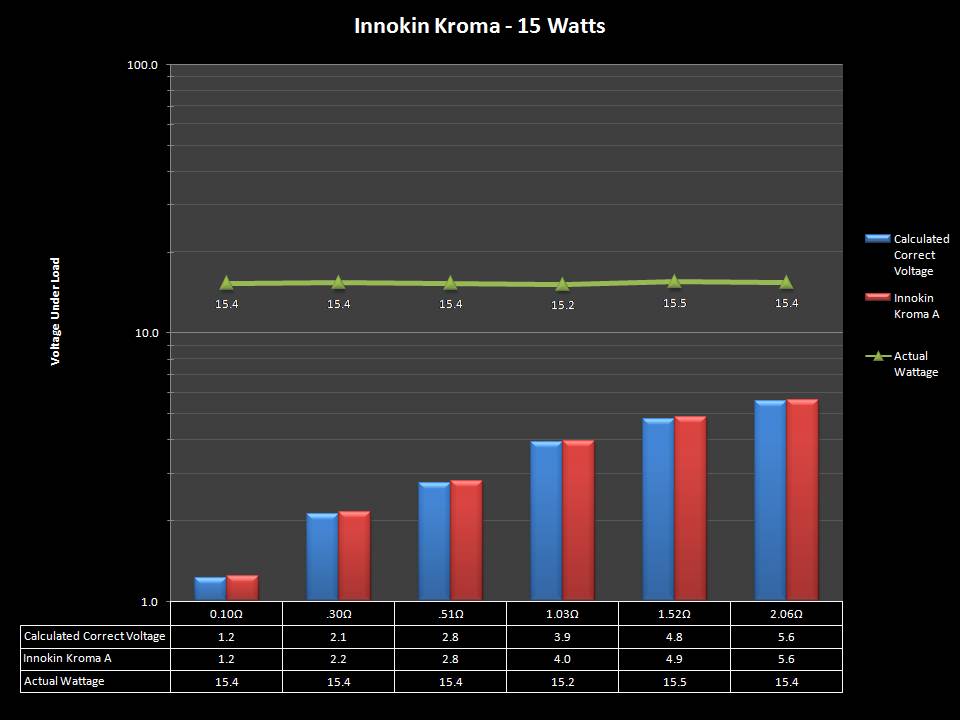
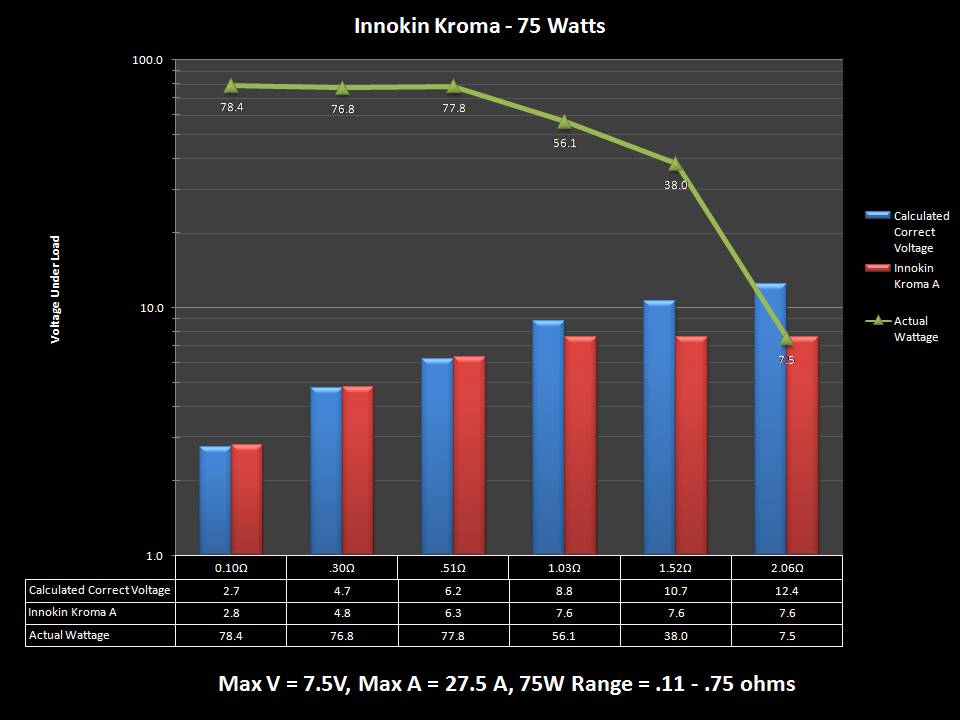
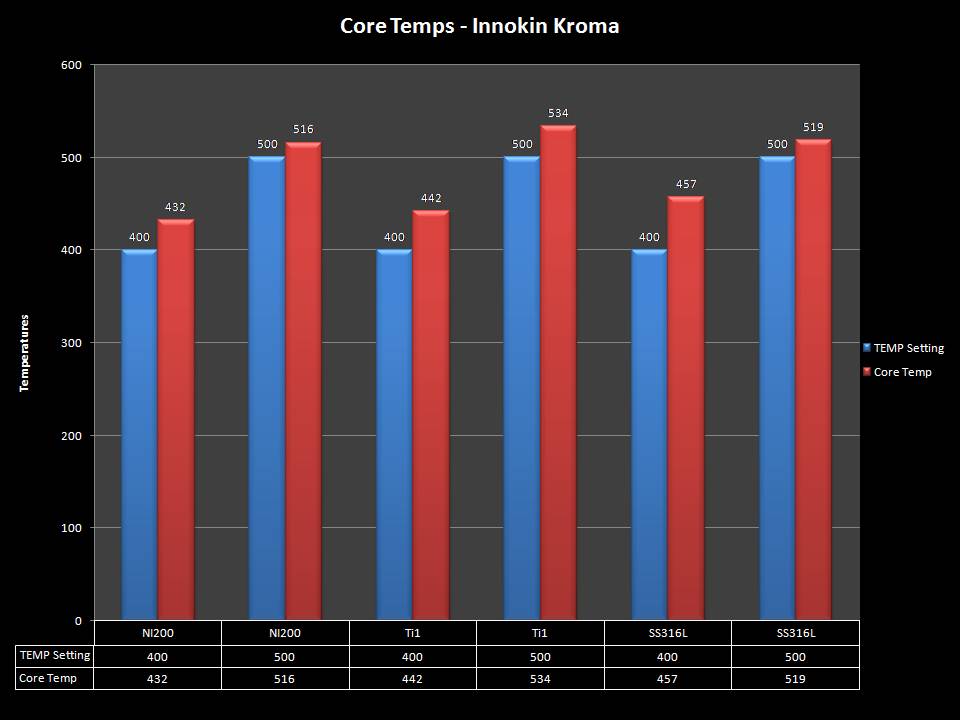
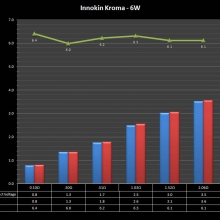
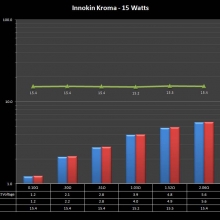
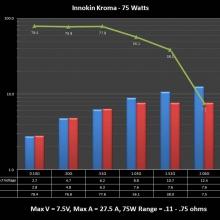
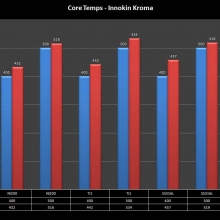
THE INNOKIN KROMA-A & A NEW “NOT A” CONTEST!
A PBusardo Review – Innokin Kroma-A and a new “Not A” Contest!
In this video we get happy with some good news from the FDA, then we take a look at the Innokin Kroma-A, then we give one away!
The Links:
VTA
CASAA
AVA
SFATA
R2B Smoke Free
SEVIA-USA
Aspire
Innokin
Kanger
Smok
MyVaporStore
Smith & Baxter
Halcyon Vapors
Conviction E-Liquids
The Video:
*NOTE: Any use of these videos in part or in their entirety without Phil Busardo’s expressed written consent is strictly prohibited.
The Photos:





























































































































































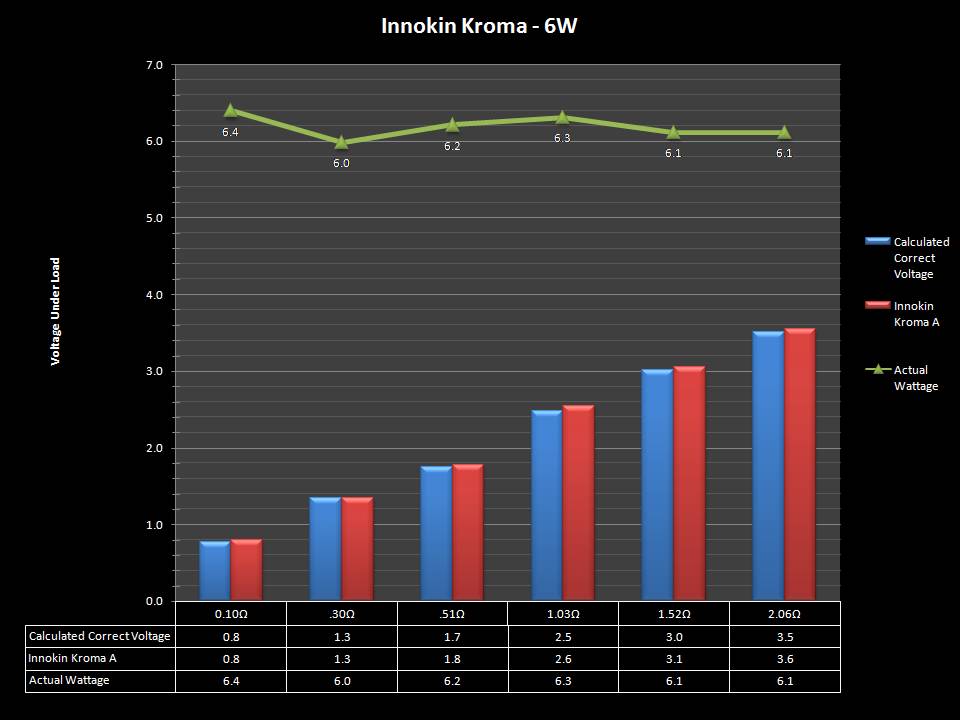
































 Store
Store












