Category: Recent News
A BATTERY MOOCH POST: Vapcell 25A 2800mAh 18650…accurately rated, a Sony VTC5D rewrap
In my opinion this is a rewrap of the great performing Sony VTC5D, appearing and performing identically to the Sony. It performs slightly better than the VTC5A but not quite as well as the VTC6A.
I am estimating this cell’s ratings to be 25A and 2800mAh.
Two cells were donated for the purposes of testing by Vapcell (https://www.vapcelltech.com).
Ratings graphic: https://imgur.com/a/3JGY8t6
I want to work for the vaping community full time! If you feel what I do is worth a couple dollars a month and you would like early access to battery availability and testing news and a say in what I test then please consider becoming a patron and supporting my testing efforts: https://www.patreon.com/batterymooch
These tests only note the estimated ratings for these batteries at the time I tested them. Any battery that is not a genuine Samsung, Sony, LG, Panasonic, or Sanyo can change at any time! This is one of the hazards of using “rewrapped” batteries or batteries from other manufacturers so carefully research any battery you are considering using before purchasing.
Misusing or mishandling lithium-ion batteries can pose a SERIOUS RISK of personal injury or property damage. They are not meant to be used outside of a protected battery pack. Never exceed the battery’s continuous current rating and keep the plastic wrap and top insulating ring in perfect condition.
Any rating in my ratings tables can change at any time as different grade cells appear on the market, we get swamped with fakes, or new information becomes available to me. Please, never assume that the ratings in the tables are permanent and will never change! Always download the latest version before considering any cell purchase.
To see how other cells have tested check out this link: https://www.e-cigarette-forum.com/forum/blog-entry/list-of-battery-tests.7436/
ADVOCACY INTERVIEW FROM NVE ALABAMA – FIG RAMSEY
A PBusardo Advocacy Interview – Fig Ramsey from NVE Alabama
Fig Ramsey is the creator of VapeTithing, a former long-time smoker and the President of Feels Good Vapor, which includes the Gonzo Vapors and AdvoCotton brands.
As an active and passionate advocate for vapor products, and realizing that many of our groups are underfunded and seem to have less support than many may assume, he sought to find a solution. With an eye towards change, he developed innovative strategies wherever he does business to help fund the future.
Specifically, Fig is the inspiration behind and architect of #GonzoGives, #FundTheFuture, and #VapeTithing fundraising strategies.
These programs were designed to be based on a “percentage-of-sales” funding basis. This means that even in the areas where sales are low, his company has a mechanism for participation. This is a possibility even when sales do not support a recurring membership commitment.
Fig’s current focus is to continue to build upon the programs’ successes, leading by example, in an effort to encourage other industry business leaders, whether manufacturers or distributors, to financially participate in legitimate Advocacy and Trade Organizations.
The Video:
*NOTE: Any use of these videos in part or in their entirety without Phil Busardo’s expressed written consent is strictly prohibited.
ADVOCACY INTERVIEW FROM NVE ALABAMA – MARK ANTON
A PBusardo Advocacy Interview – Mark Anton from NVE Alabama
Mark is the new Legislative Director of SFATA (Smoke-Free Alternatives Trade Association).
Mark Anton has been involved in the vapor industry since 2008 and is CEO and founder of What A Smoke, LLC an e-cig and vapor device manufacturer and distributor. Mark also served as a principal investigator on a government contract to develop a standardized e-cigarette for research. In addition to his work with SFATA, Mr. Anton serves as Legislative Director for federal matters for the New Jersey Vapor Rights Coalition (NJVRC) which represents retailers in his home state. Mark is passionate about the industry and has testified before the FDA on vapor matters and works with multiple associations to promote the vapor space before legislative bodies. Anton served nearly two terms on the SFATA Board of Directors prior to assuming his role as Executive Director; a significant portion of his tenure in the role of Vice-President.
Mark’s Email Address: Mark@sfata.org
The Video:
*NOTE: Any use of these videos in part or in their entirety without Phil Busardo’s expressed written consent is strictly prohibited.
ADVOCACY INTERVIEW FROM NVE ALABAMA – GREG TROUTMAN
A PBusardo Advocacy Interview – Greg Troutman from NVE Alabama
Greg is a Louisville-based attorney who represents various national and international interests in the vaping industry. He also engages in advocacy and political efforts on behalf of the industry.
Greg’s Email Address: jgtatty@yahoo.com
The Video:
*NOTE: Any use of these videos in part or in their entirety without Phil Busardo’s expressed written consent is strictly prohibited.
FROM TONY ABBOUD/VTA – FEDERAL LEGISLATIVE UPDATE!

NO COLE-BISHOP LANGUAGE IN SENATE APPROPRIATIONS BILL;
FLAVOR AMENDMENT AVERTED
May 25, 2018
As we reported last week, the House Appropriations Committee included language in the House Agriculture Appropriations bill that gave regulatory relief only for combustible tobacco products. As we also noted, our teams were working to ensure that such language would not be included in the Senate Agriculture Appropriations bill.
This week, no such language was included in the Chairman’s mark, nor offered as an amendment either at the Senate Agriculture Appropriations subcommittee (on Tuesday) or by the full Senate Appropriations Committee (yesterday). The bill as marked up contains no language that would give relief to combustible tobacco products. So, for now, we have prevented the House’s language from being included in the Senate bill.
At the same time, our teams were working against an effort to add legislative language regarding flavors to the Senate bill. Our efforts in this regard also were successful and the proponent, Sen. Richard Durbin (D-IL), agreed not to offer any such amendment. Instead, Sen. Durbin was able to get “report language” inserted to the Committee report. Report language is not law, is not precedent and agencies, like the FDA, tend to treat this language as advisory. It is not in any way legally binding on any Agency or entity.
Below is the report language that was included in the Senate Appropriations report in lieu of Sen. Durbin moving forward with an amendment seeking to ban flavors. As you will see, the focus of the report language is on youth, marketing and flavors, issues which VTA is tackling head on, and issues that FDA has already announced they are pursuing.
Youth Tobacco Use Prevention. – While FDA has recently announced a new Youth Tobacco Prevention Plan to attempt to curb the use of e-cigarettes among youth, the Committee is concerned with the irresponsible marketing by some manufacturers, as well as the role characterizing flavors play in youth initiation of tobacco products. In March 2018, FDA issued an Advanced Notice of Proposed Rulemaking to examine regulatory options for tobacco product flavorings. The Committee strongly encourages the Agency to complete the regulatory process in an expeditious manner, ideally within one year, and in a way that supports prevention of youth tobacco initiation. The Agency is instructed to provide the Committee with a timeframe for when the regulatory process will be completed. Additionally, the Committee is concerned that FDA is not fully enforcing their prohibition of new or changed e-cigarettes and other nicotine products after August 8, 2016, without prior FDA review and authorization. Therefore, the Committee directs FDA to order the removal of any “deemed tobacco products” introduced after the August 8, 2016 deadline without first seeking the required FDA authorization. Finally, the Committee is concerned about the lack of adequate age verification rules to prevent internet sales of e-cigarette tobacco products to children, and directs FDA to establish these rules within one year, both at the time of sale and delivery of the product.
At best, the report language affirms what the FDA has already announced they are doing. Importantly, report language has been used many times in the past when there was no support for actual legislation. Such language often asks an agency to “report” back to Congress on certain items. In our experience, it is only on the rarest of occasions that any agency either reports back or, if they do at all, reports back within the timeframe requested.
Furthermore, these questions could have been addressed at the budget hearing earlier this spring when Commissioner Gottlieb testified at the Senate Appropriations Committee. But, they were not.
Sen. Durbin’s brief speech yesterday at the full committee markup attempted to elicit supposed horrors associated with nicotine even though Commissioner Gottlieb and Director Zeller repeatedly state publicly that nicotine is not the problem. Moreover, in his zeal to castigate the vapor industry to the “hottest ring in hell,” Sen. Durbin went so far as to equate nicotine addiction to opioid addiction. Even if offered to score political points, such comments do a grave disservice to all those teens and adults who are suffering and dying from opioids and demonstrate a callousness to all the family members left to pick up the pieces of what is a true addiction crisis confronting our country.
Moving forward, we are charting a path for educating and informing Members of Congress over the perils associated with the House’s appropriations language and the importance of supporting vapor products which are challenging the dominance of combustible tobacco products in the marketplace.
To that end, please take a moment to register for VAPE & THE FDA 3, VTA’s national conference and Day on Capitol Hillwhich will be held on June 26-27, 2018. Now more than ever, your voice needs to be heard. Lean more and register at our conference registration portal by CLICKING HERE!
Thank you for all you do to defend vapor!

Tony Abboud
Executive Director
Vapor Technology Association
ADVOCACY INTERVIEW FROM NVE ALABAMA – GREG CONLEY
A PBusardo Advocacy Interview – Greg Conley from NVE Alabama
The Links:
American Vaping Association – AVA
The Video:
*NOTE: Any use of these videos in part or in their entirety without Phil Busardo’s expressed written consent is strictly prohibited.
A BATTERY MOOCH POST: Cylaid Yellow 60A 3100mAh 18650…Sony VTC6 rewrap with preposterous pulse rating
In my opinion this is a rewrap of the Sony VTC6, appearing and performing identically to the Sony. Because of the hugely exaggerated ratings I am giving this cell a Do Not Buy recommendation.
This Cylaid’s 40A continuous rating is hugely exaggerated as Sony rates the VTC6 at 15A continuous. Operating at higher current levels is mentioned in the datasheet but you must not exceed its 80°C maximum temperature rating. Rating this cell at 40A is outrageous.
This Cylaid’s 60A “pulse” rating is useless as we don’t know the width of the pulses, the time between them, and the criteria used to set the rating.
The Sony VTC6 is rated at 3000mAh so this Cylaid’s 3100mAh capacity is exaggerated too.
I am estimating this cell’s ratings to be the same as Sony’s ratings for the VTC6; 15A continuous, higher if not allowed to reach 75°C, and 3000mAh.
Two cells were purchased for the purposes of testing by me.
Ratings graphic: https://imgur.com/a/JdEZvMu
I want to work for the vaping community full time! If you feel what I do is worth a couple dollars a month and you would like early access to battery availability and testing news and a say in what I test then please consider becoming a patron and supporting my testing efforts: https://www.patreon.com/batterymooch
These tests only note the estimated ratings for these batteries at the time I tested them. Any battery that is not a genuine Samsung, Sony, LG, Panasonic, or Sanyo can change at any time! This is one of the hazards of using “rewrapped” batteries or batteries from other manufacturers so carefully research any battery you are considering using before purchasing.
Misusing or mishandling lithium-ion batteries can pose a SERIOUS RISK of personal injury or property damage. They are not meant to be used outside of a protected battery pack. Never exceed the battery’s continuous current rating and keep the plastic wrap and top insulating ring in perfect condition.
Any rating in my ratings tables can change at any time as different grade cells appear on the market, we get swamped with fakes, or new information becomes available to me. Please, never assume that the ratings in the tables are permanent and will never change! Always download the latest version before considering any cell purchase.
To see how other cells have tested check out this link: https://www.e-cigarette-forum.com/forum/blog-entry/list-of-battery-tests.7436/
A BATTEEY MOOCH POST: Cylaid Purple 40A 3000mAh 18650…Samsung 30Q rewrap with useless pulse rating
In my opinion this is a rewrap of the Samsung 30Q, appearing and performing identically to the Samsung.
This Cylaid’s 25A continuous rating is exaggerated as Samsung rates the 30Q at 15A continuous. Operating at 20A is mentioned in the datasheet but you must not exceed its 75°C maximum temperature rating.
This Cylaid’s 40A “pulse” rating is useless as we don’t know the width of the pulses, the time between them, and the criteria used to set the rating.
I am estimating this cell’s ratings to be the same as Samsung’s ratings for the 30Q; 15A continuous, 20A if not allowed to reach 75°C, and 3000mAh.
Two cells were purchased for the purposes of testing by me.
Ratings graphic: https://imgur.com/a/O2O4kmi
I want to work for the vaping community full time! If you feel what I do is worth a couple dollars a month and you would like early access to battery availability and testing news and a say in what I test then please consider becoming a patron and supporting my testing efforts: https://www.patreon.com/batterymooch
These tests only note the estimated ratings for these batteries at the time I tested them. Any battery that is not a genuine Samsung, Sony, LG, Panasonic, or Sanyo can change at any time! This is one of the hazards of using “rewrapped” batteries or batteries from other manufacturers so carefully research any battery you are considering using before purchasing.
Misusing or mishandling lithium-ion batteries can pose a SERIOUS RISK of personal injury or property damage. They are not meant to be used outside of a protected battery pack. Never exceed the battery’s continuous current rating and keep the plastic wrap and top insulating ring in perfect condition.
Any rating in my ratings tables can change at any time as different grade cells appear on the market, we get swamped with fakes, or new information becomes available to me. Please, never assume that the ratings in the tables are permanent and will never change! Always download the latest version before considering any cell purchase.
To see how other cells have tested check out this link: https://www.e-cigarette-forum.com/forum/blog-entry/list-of-battery-tests.7436/
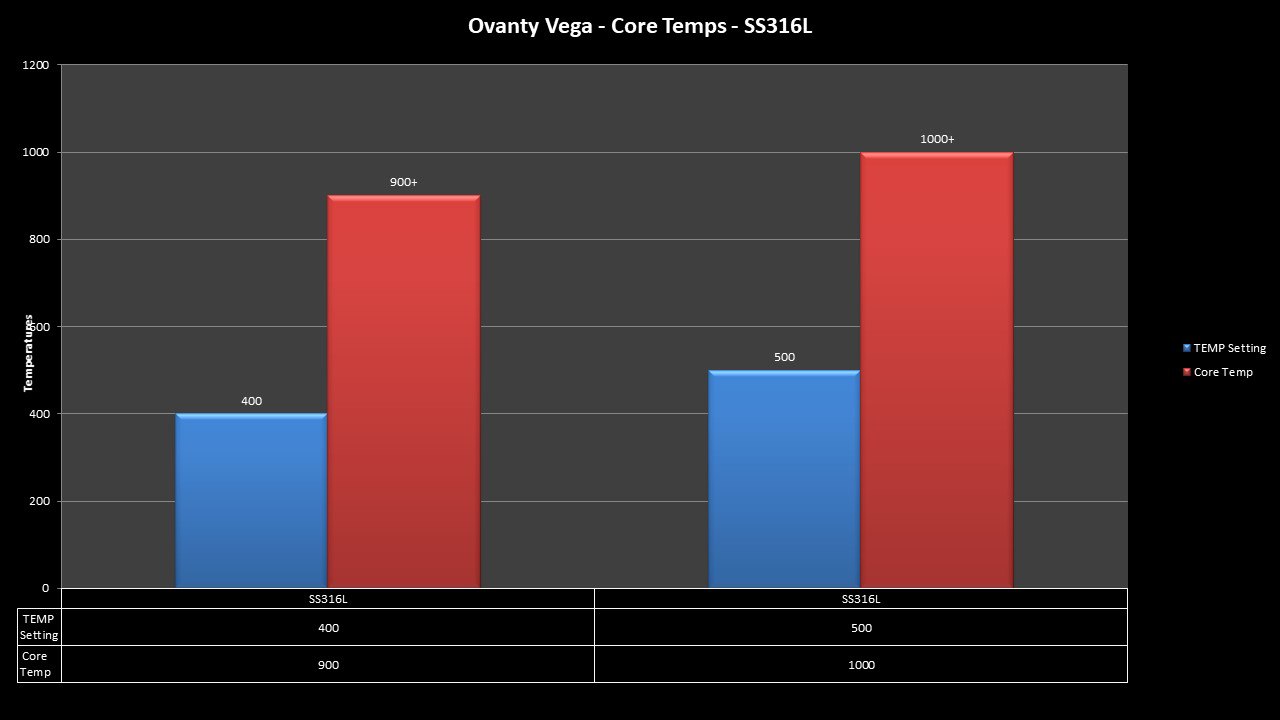
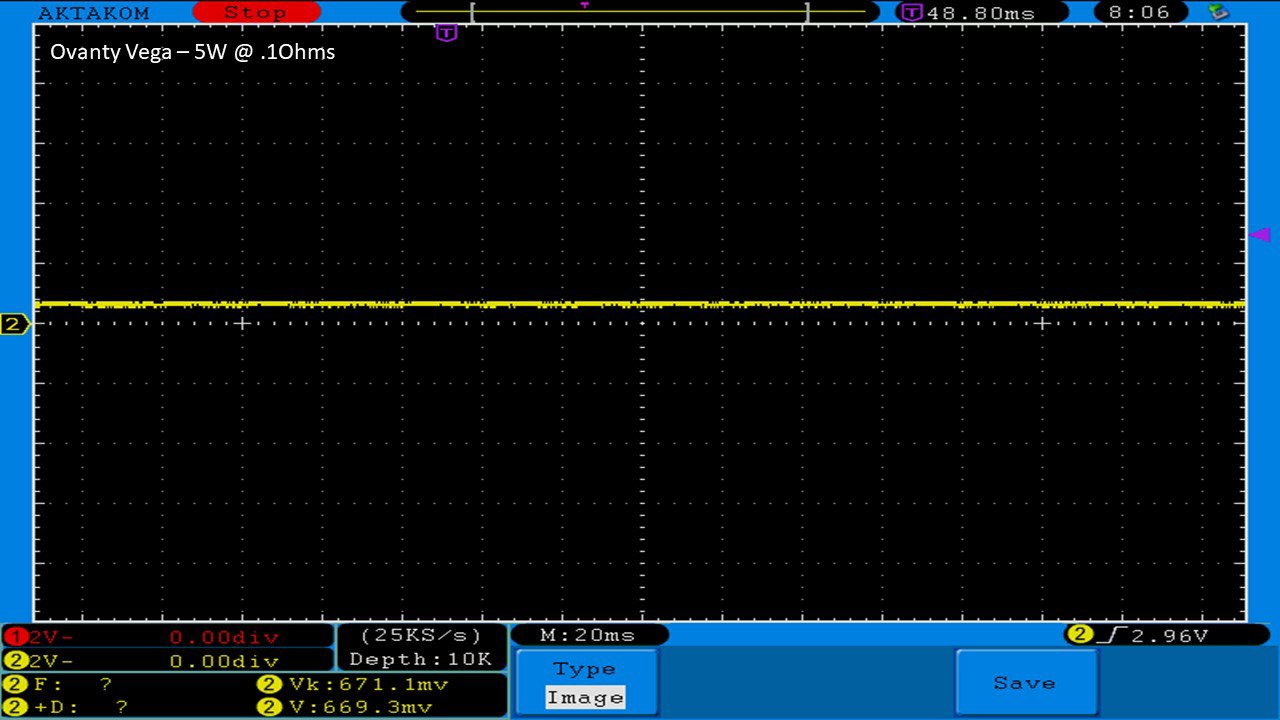
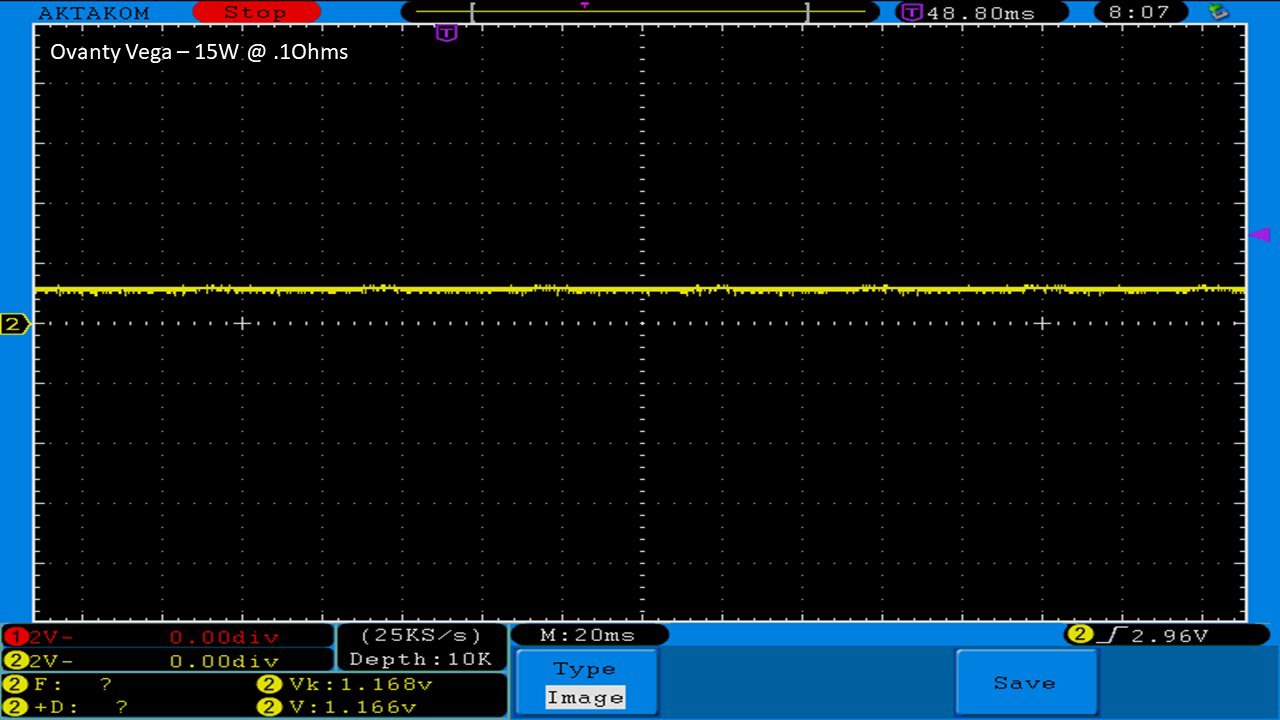
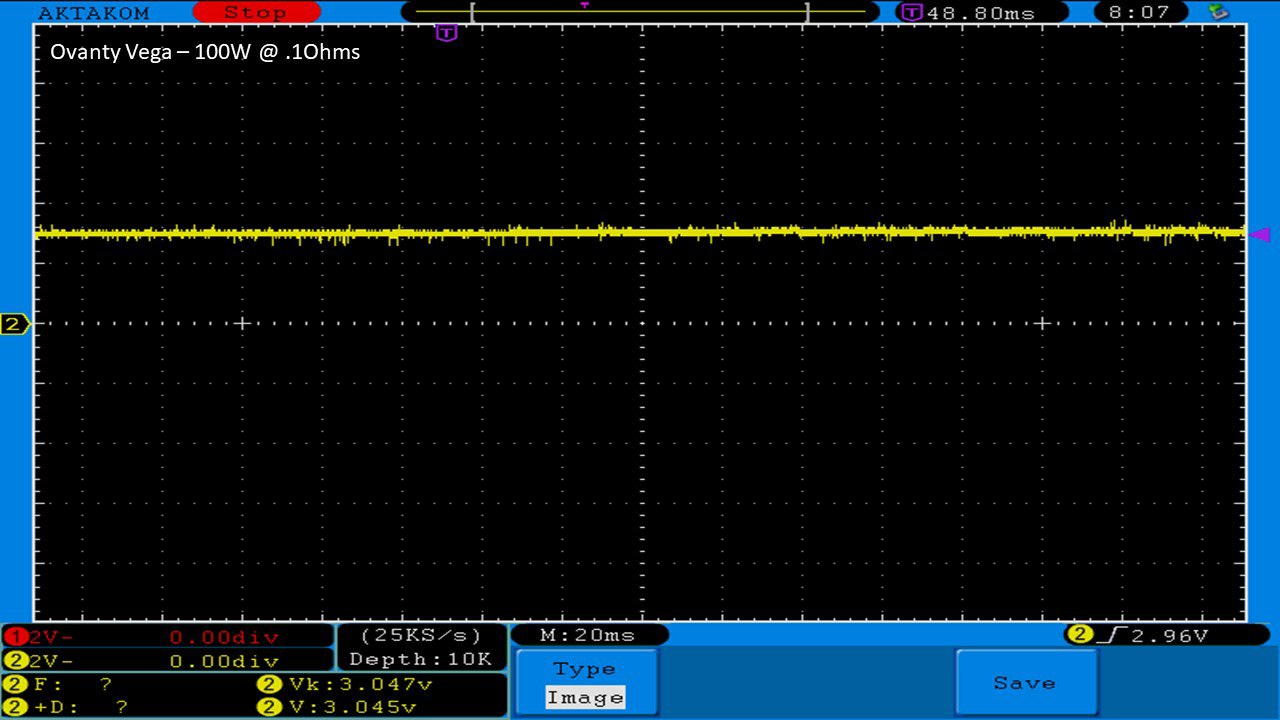
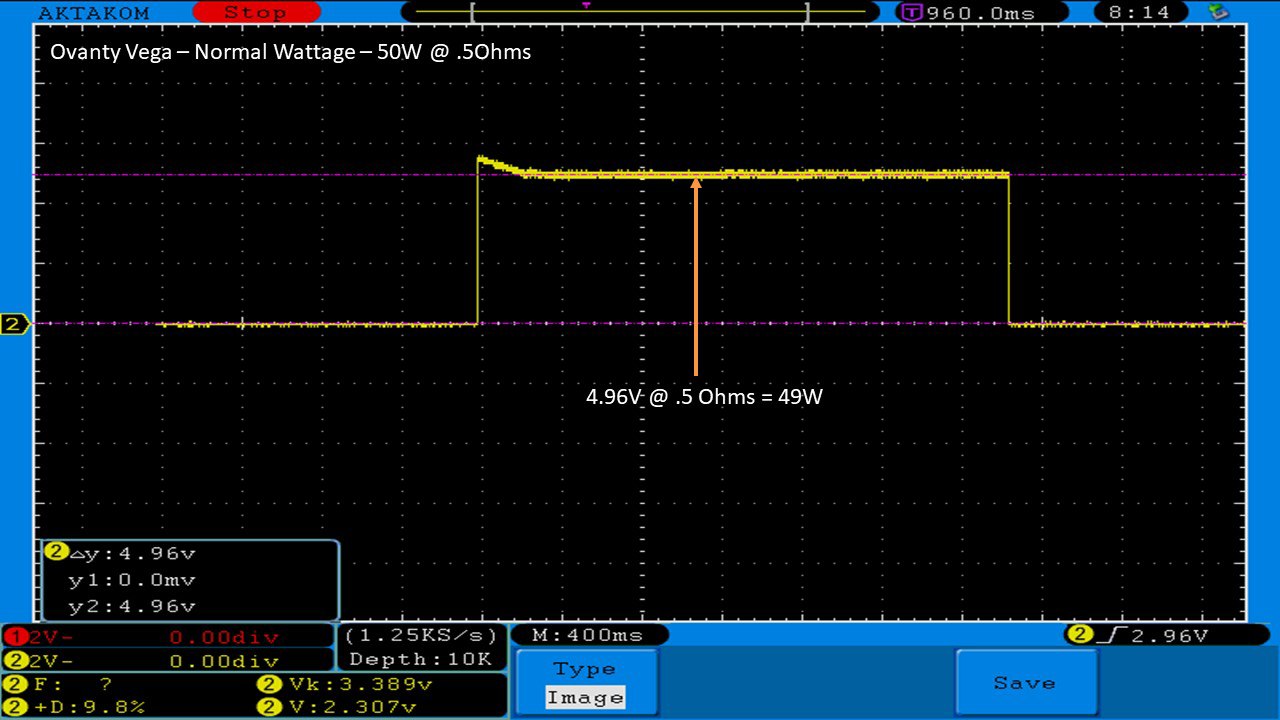
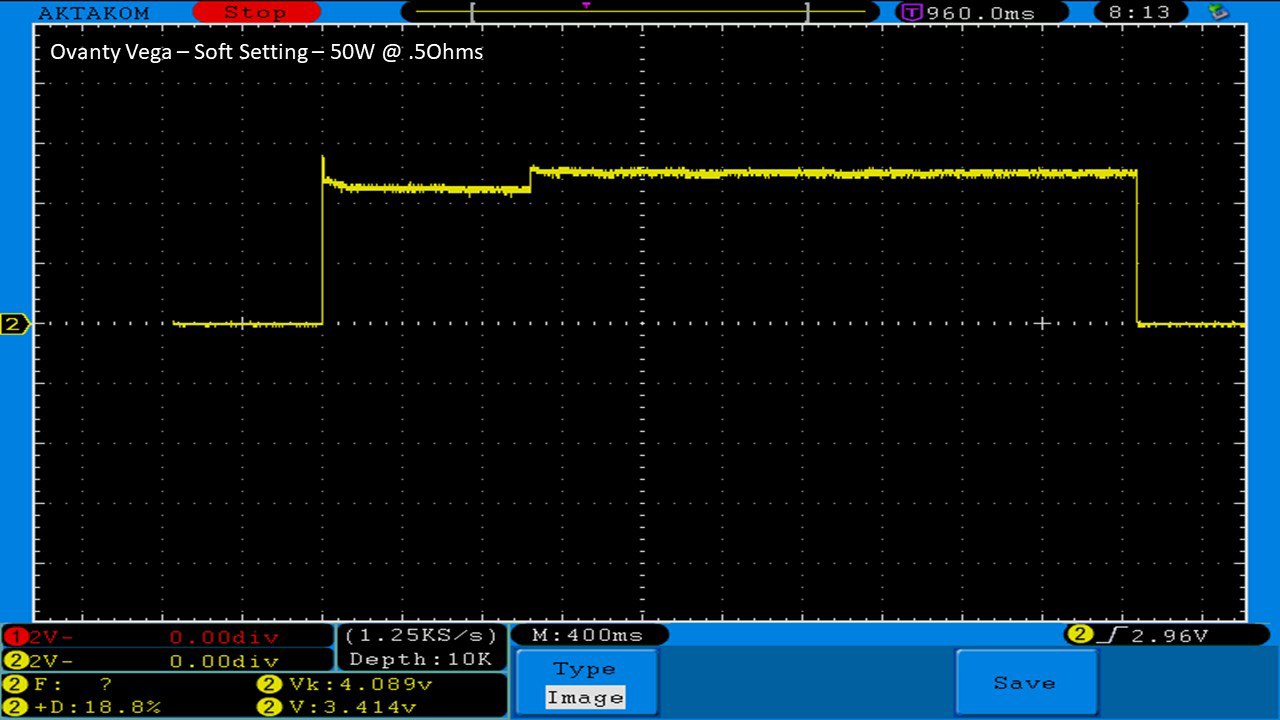
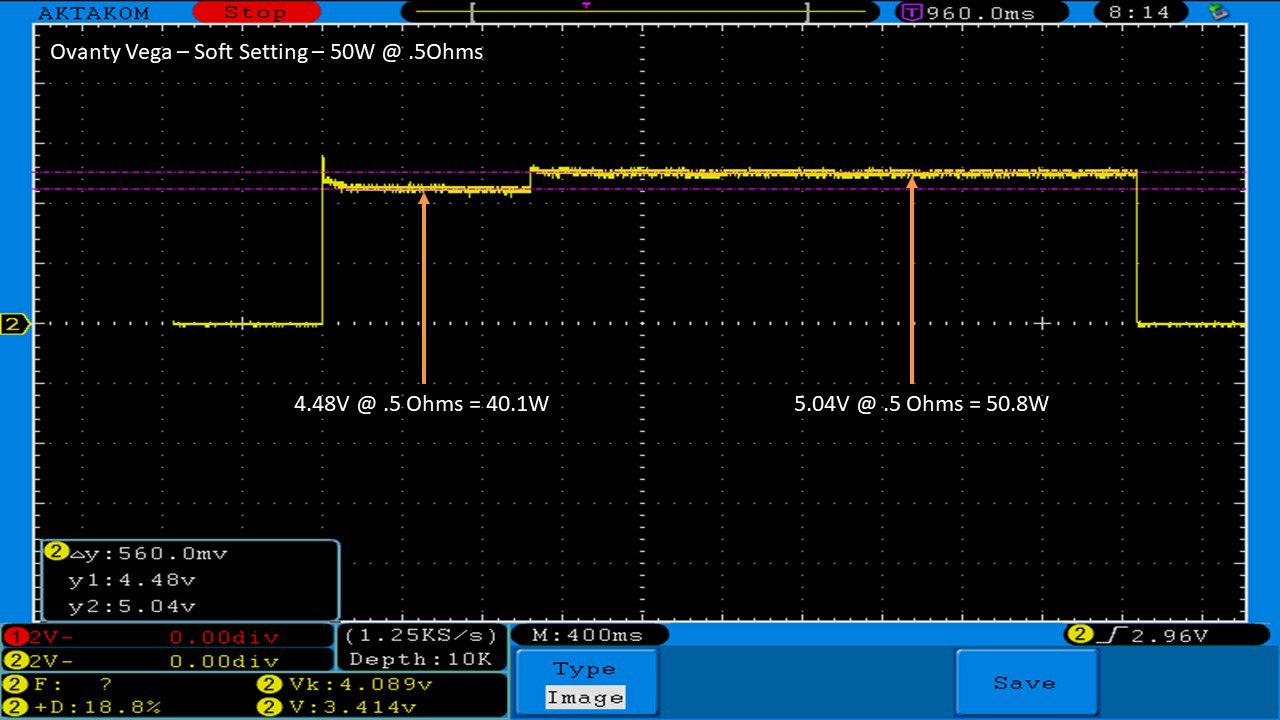
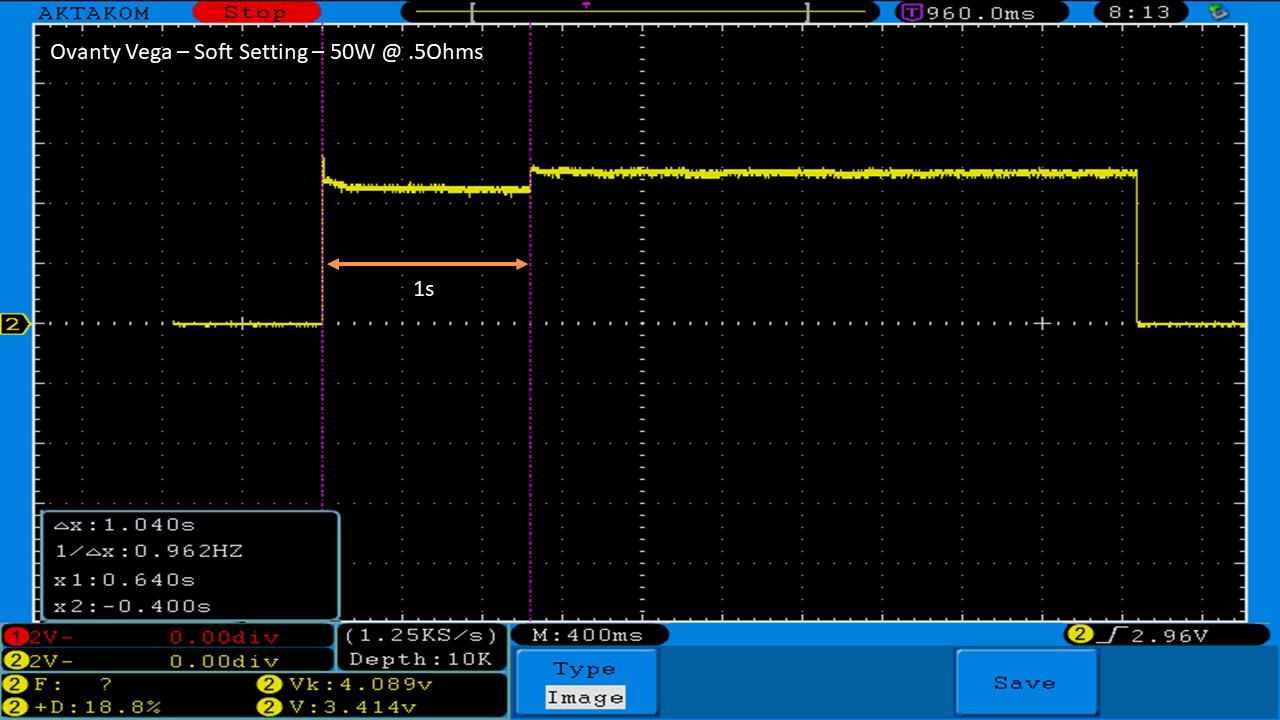
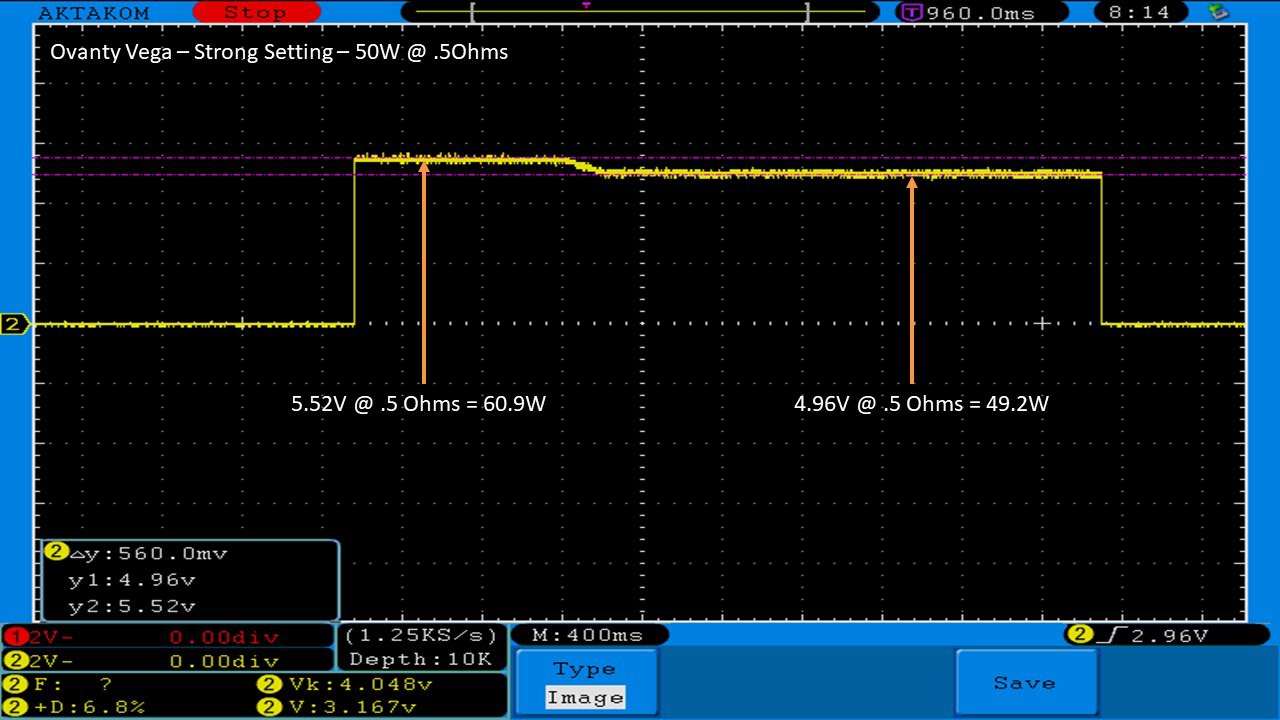
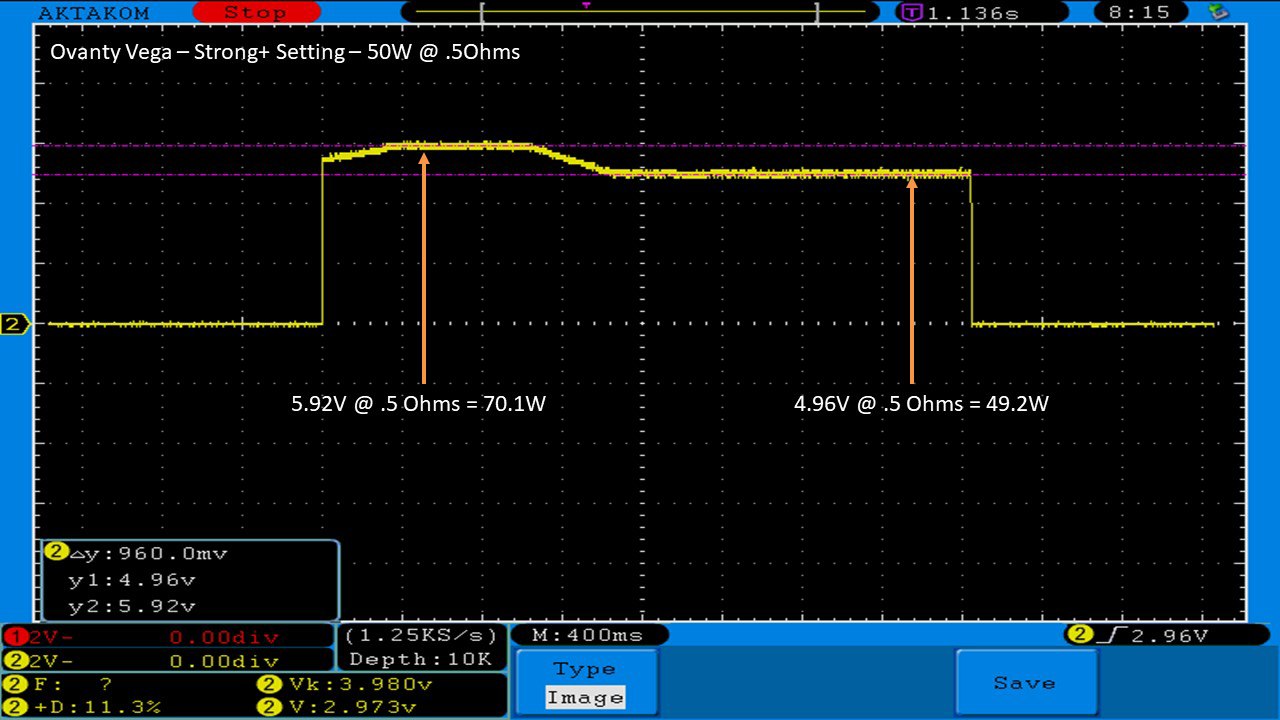
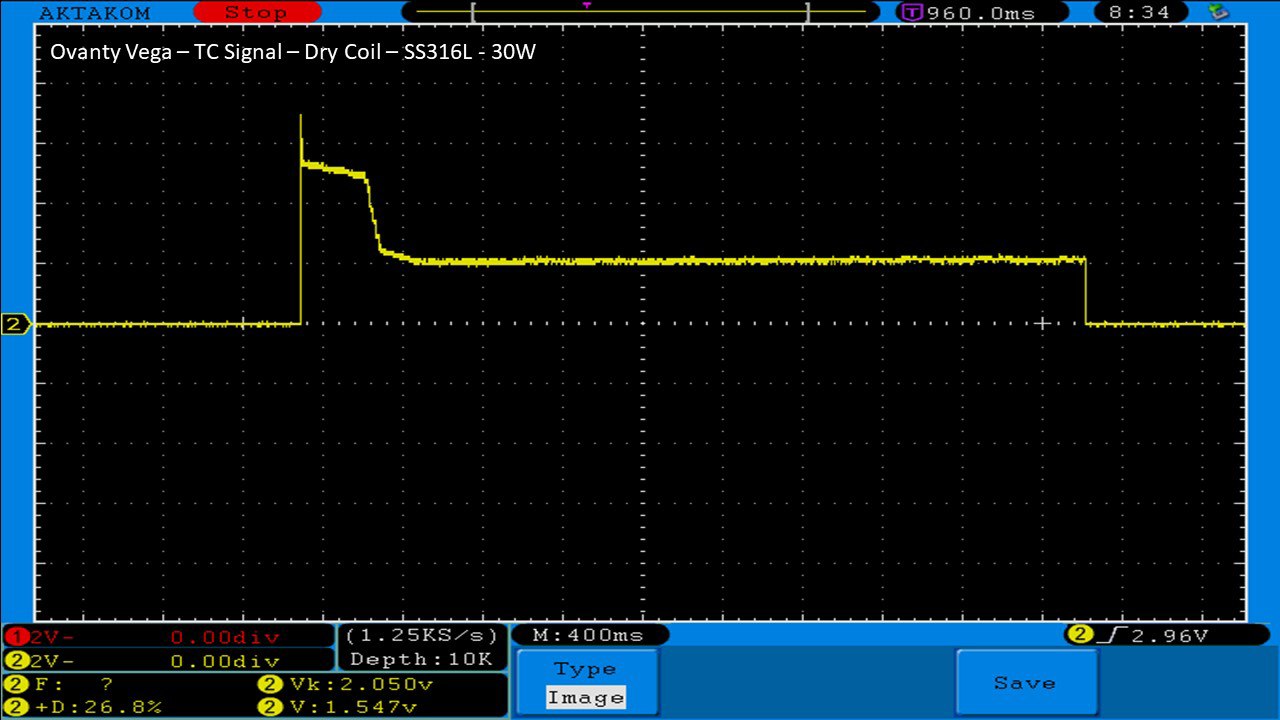
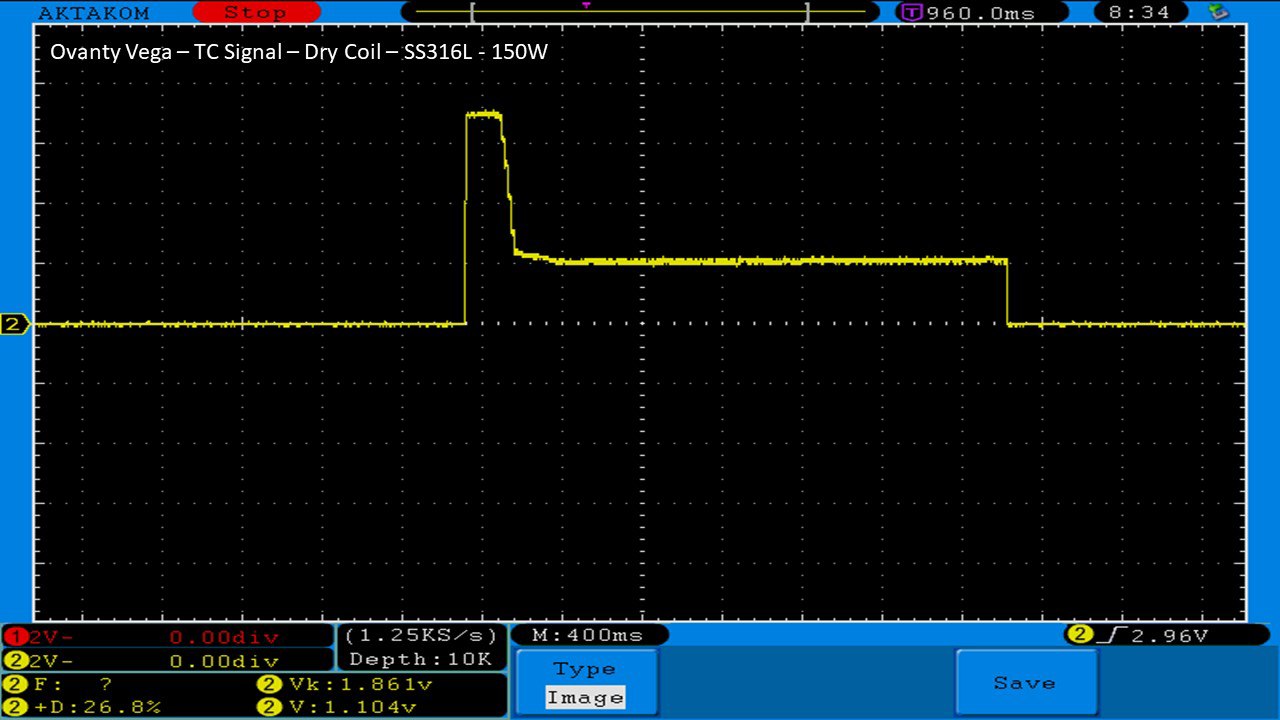
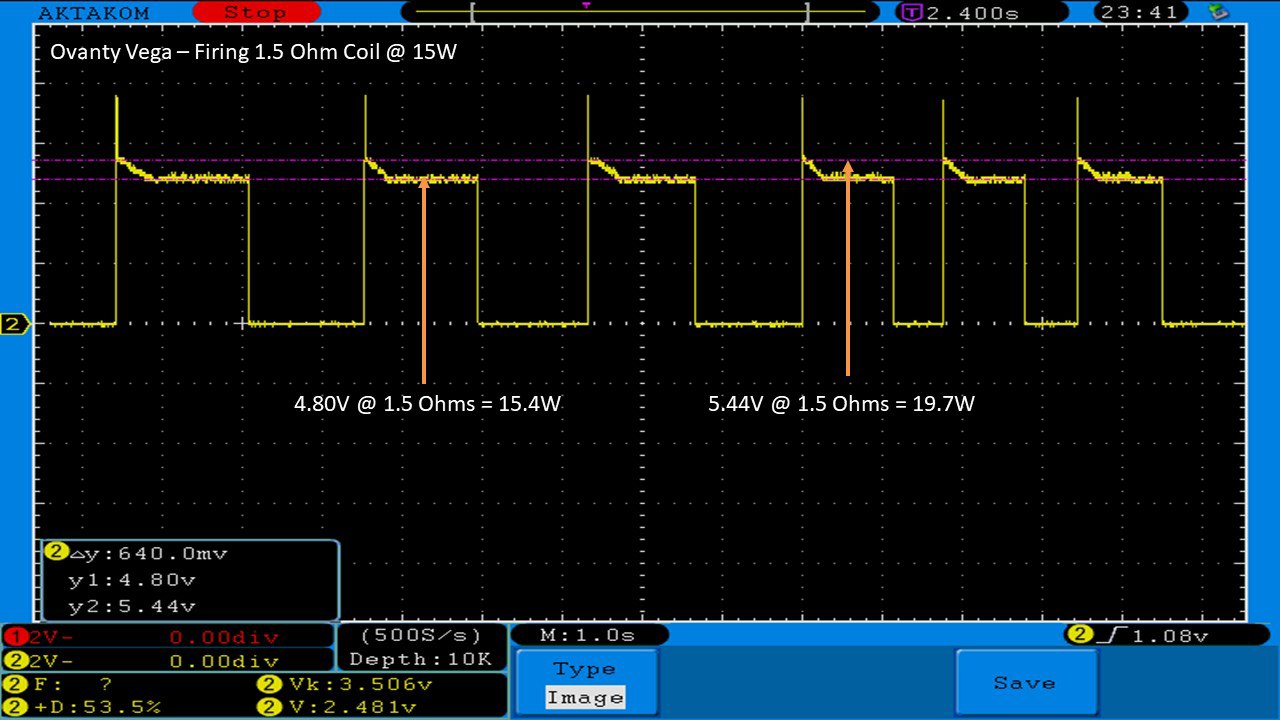
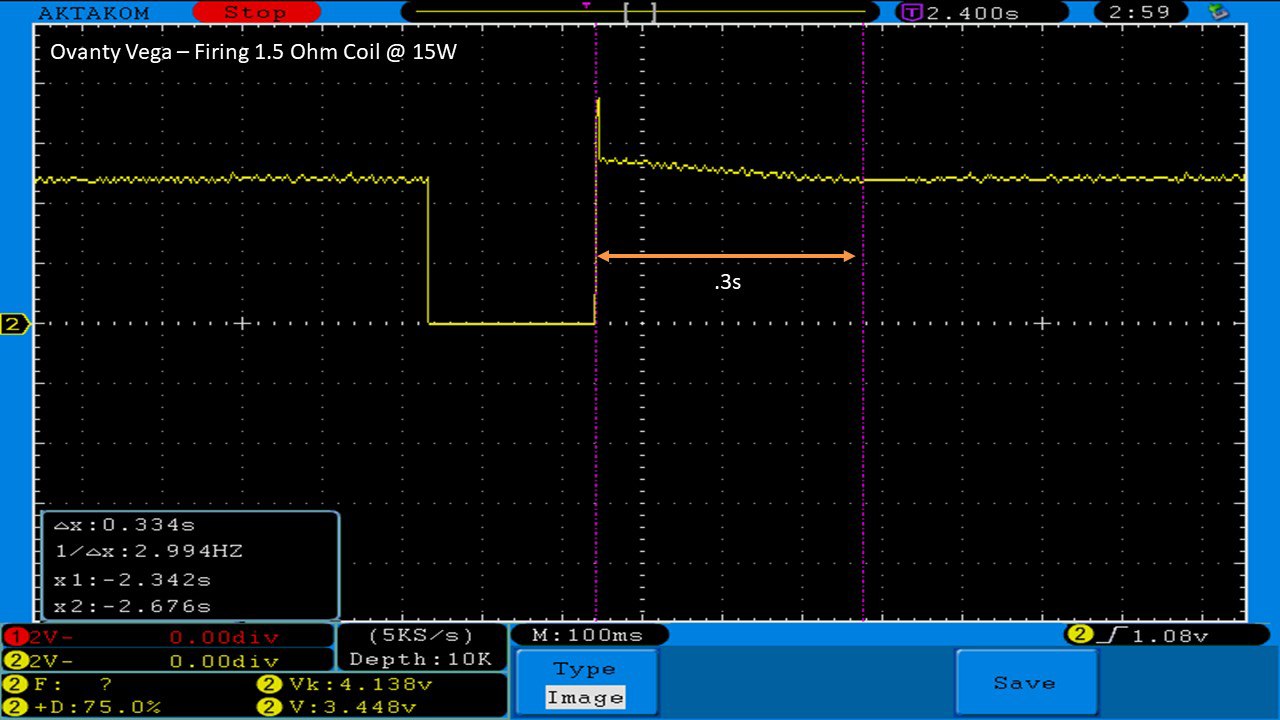
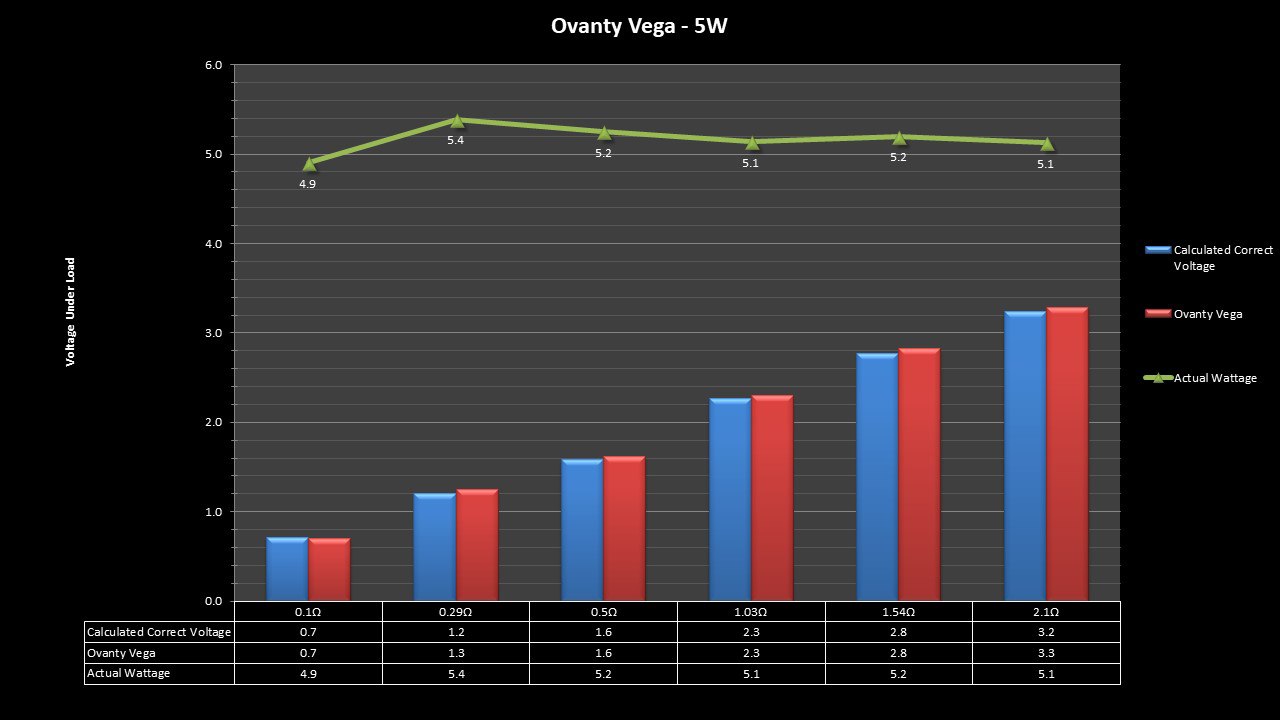
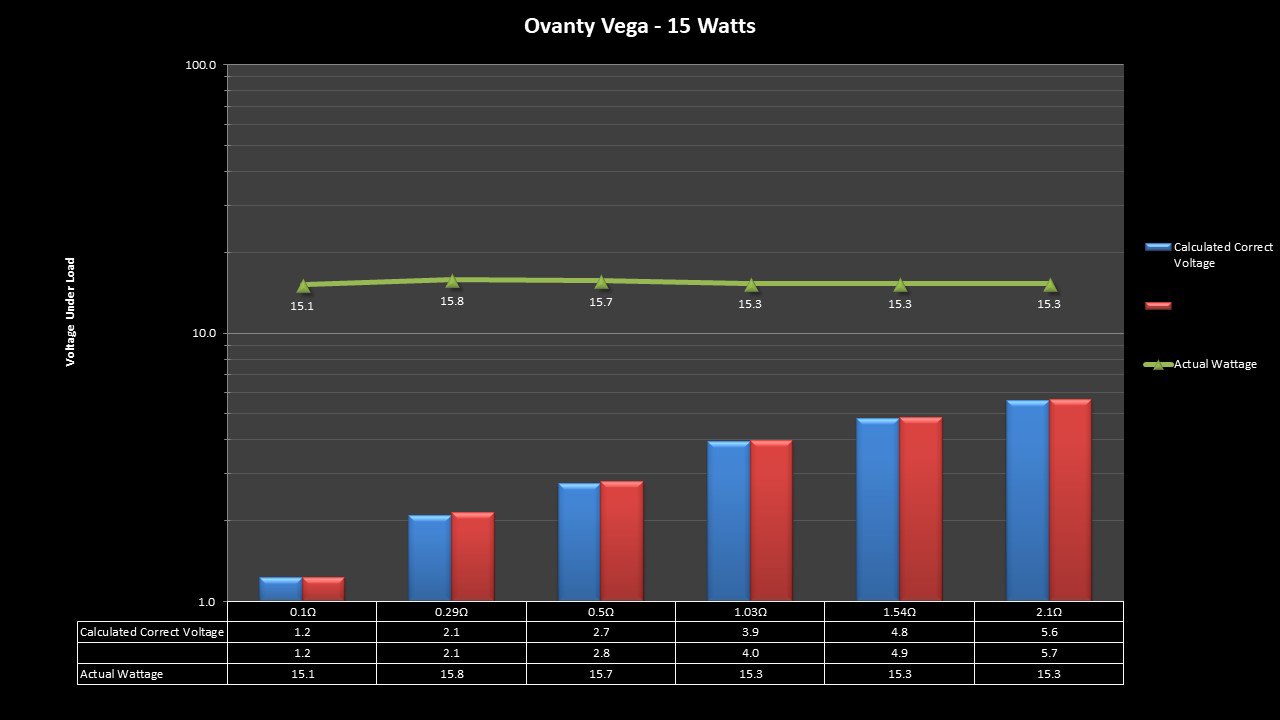
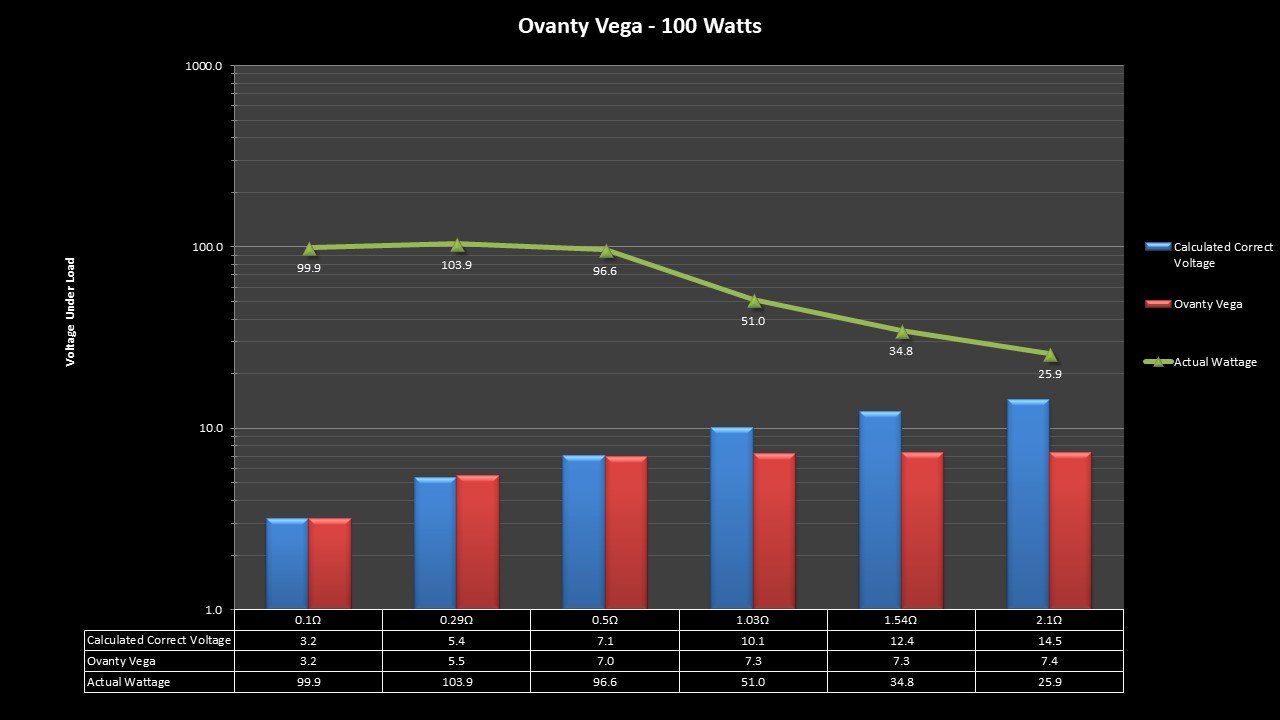
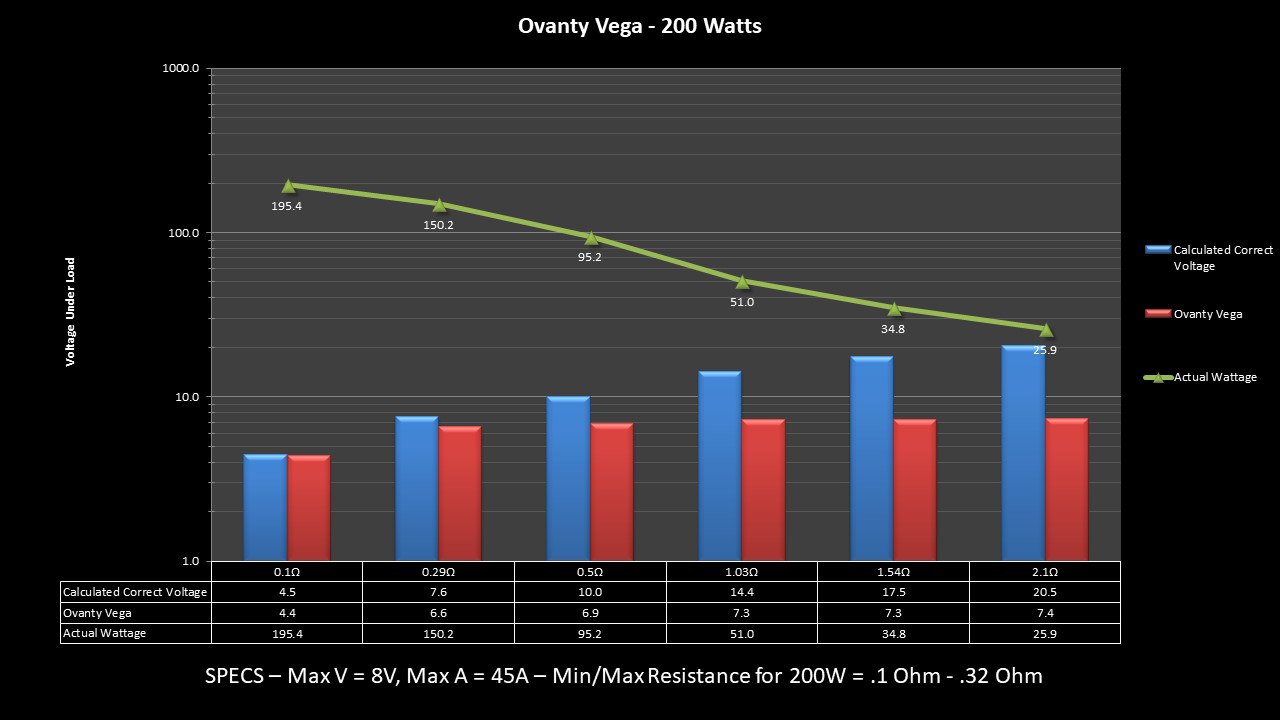
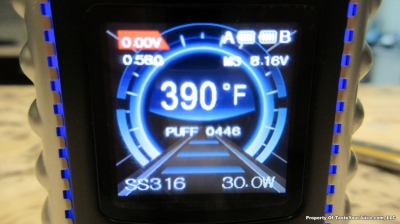
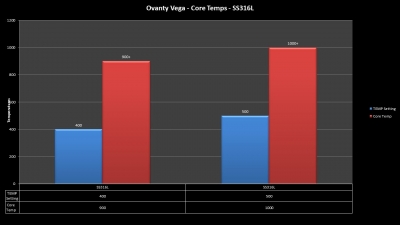
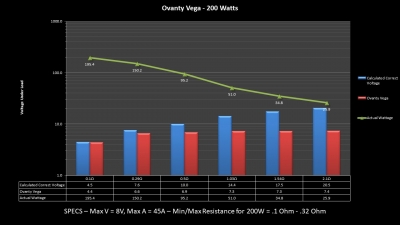
THE OVANTY VEGA & LAST CONTEST WINNER AND NEW CONTEST!
A PBusardo Review – The Oventy Vega + Last Contest Winner & New Contest!
In this video we talk a little bit about NVE Alabama, I shout out a few e-liquids, and do an interview with “The Rodman” from Vape Radio.
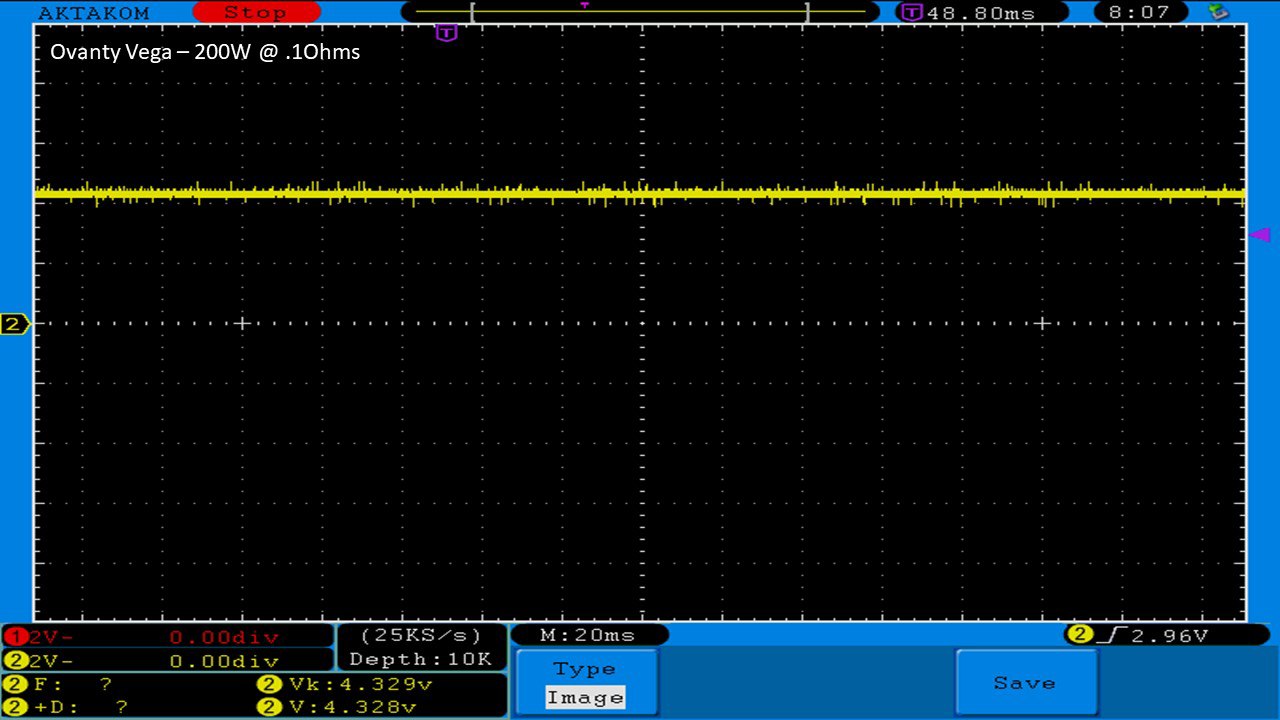
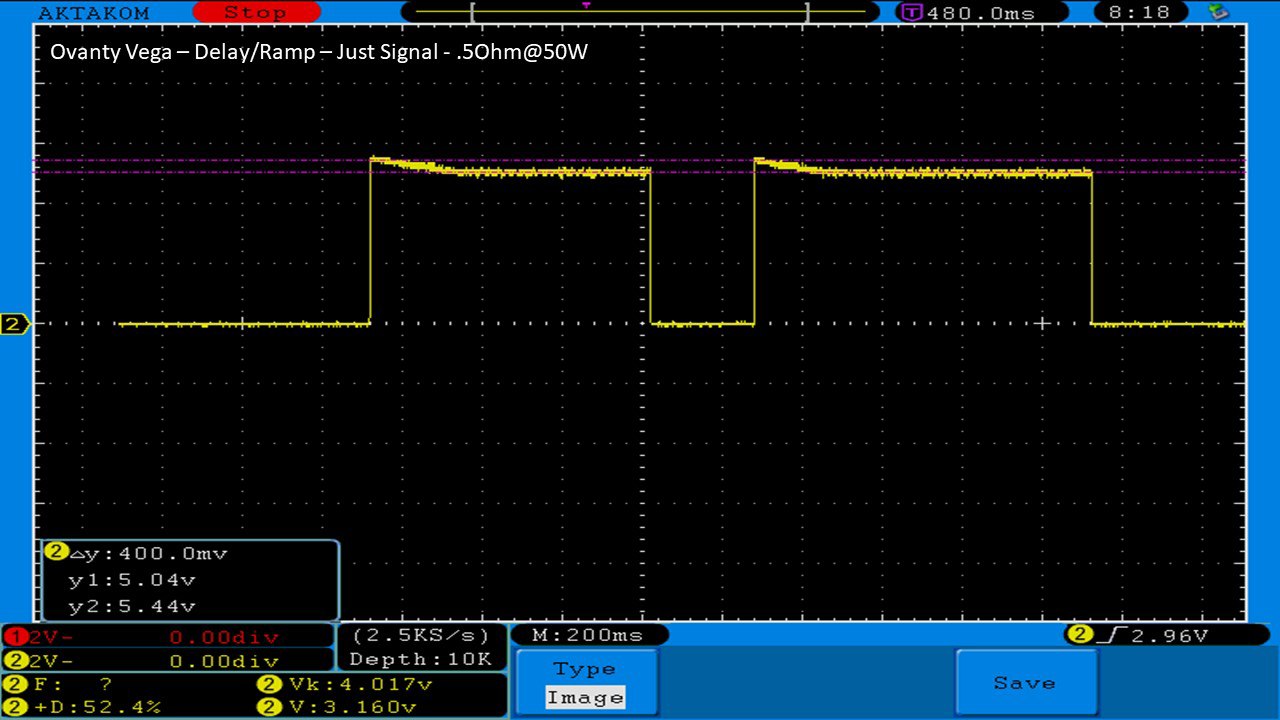
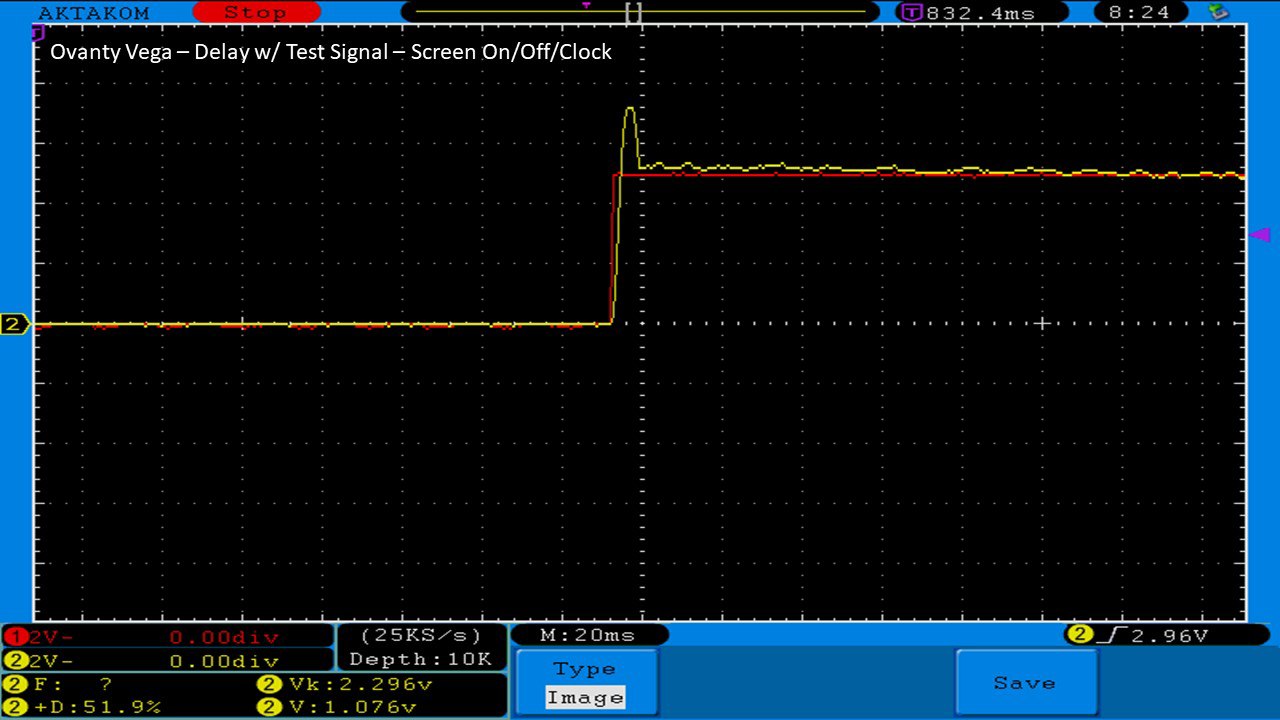
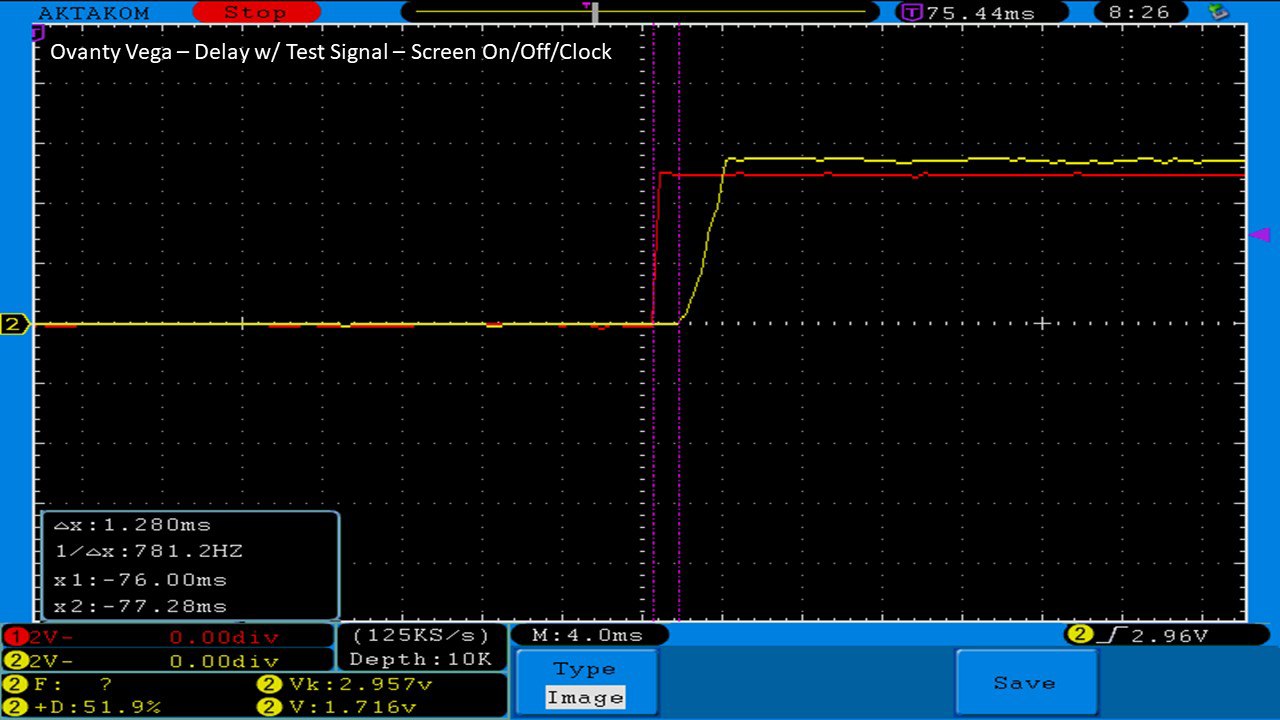
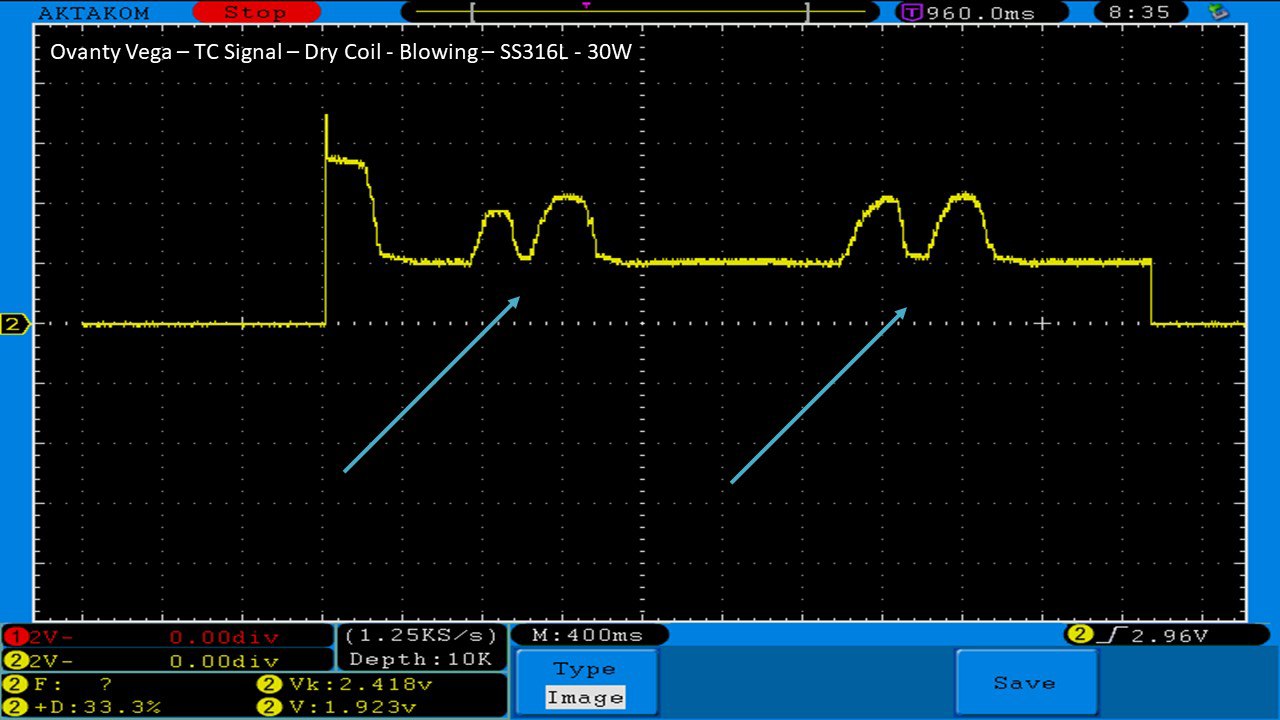
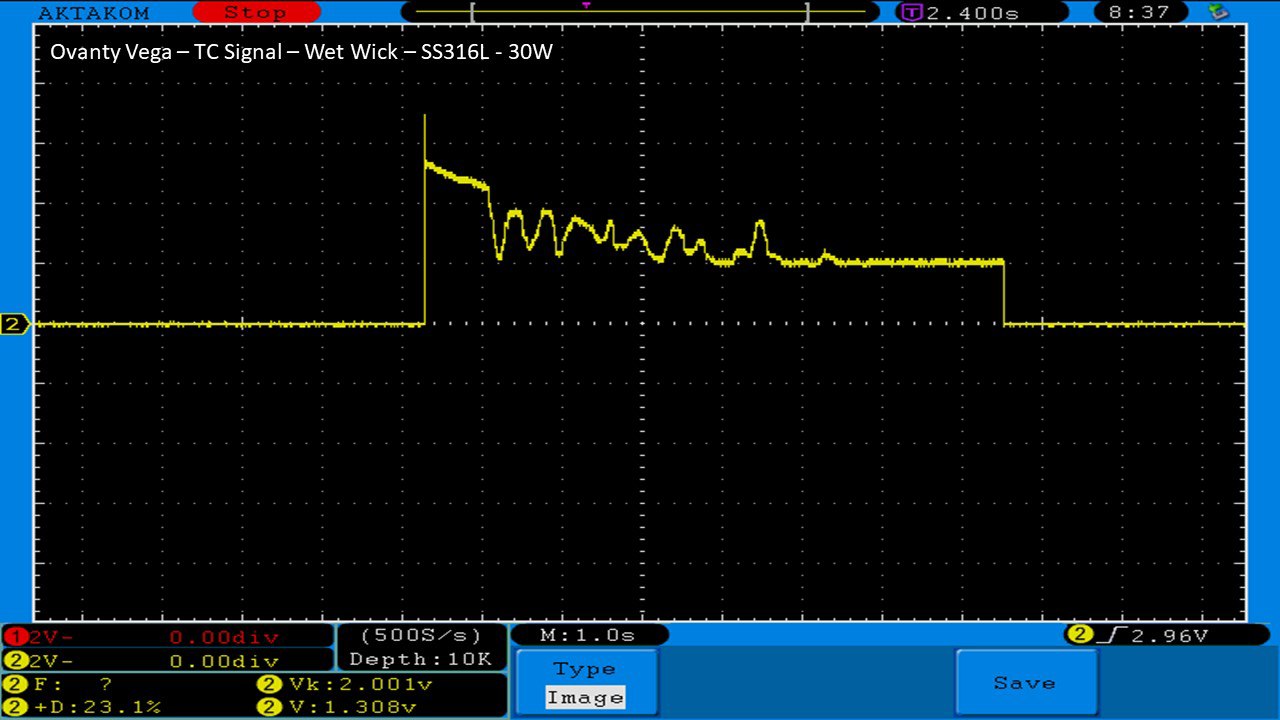
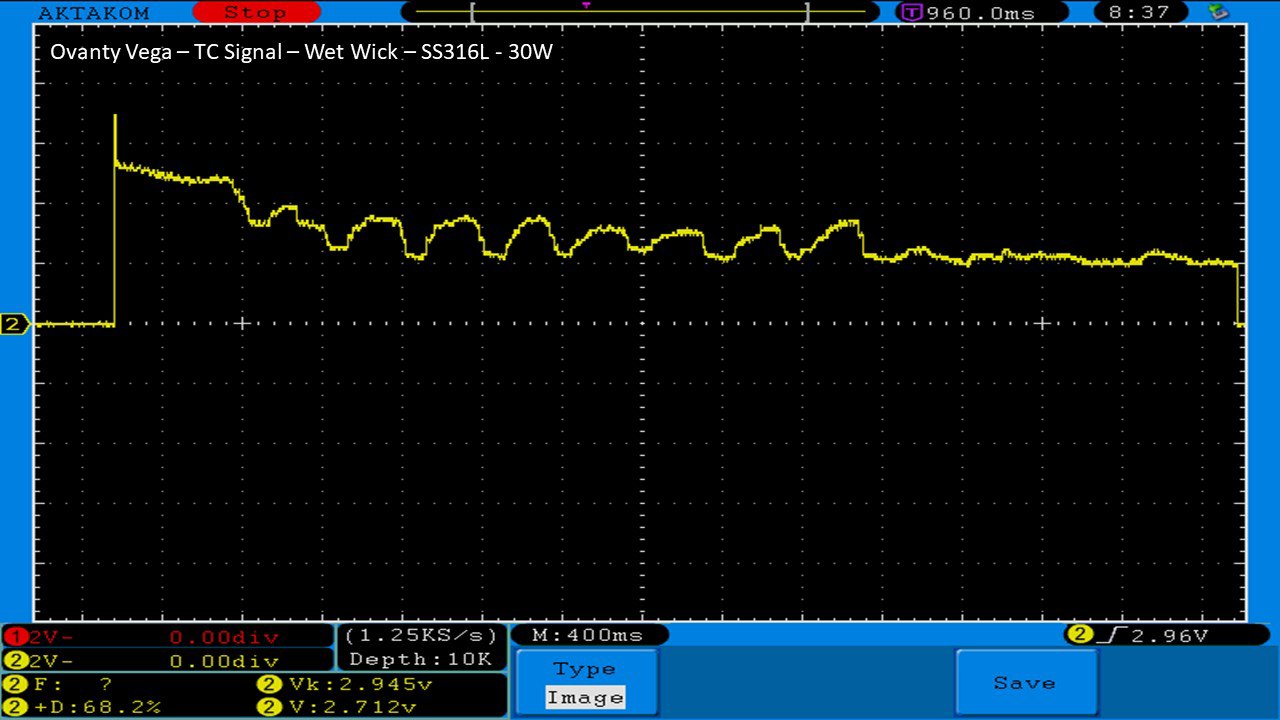
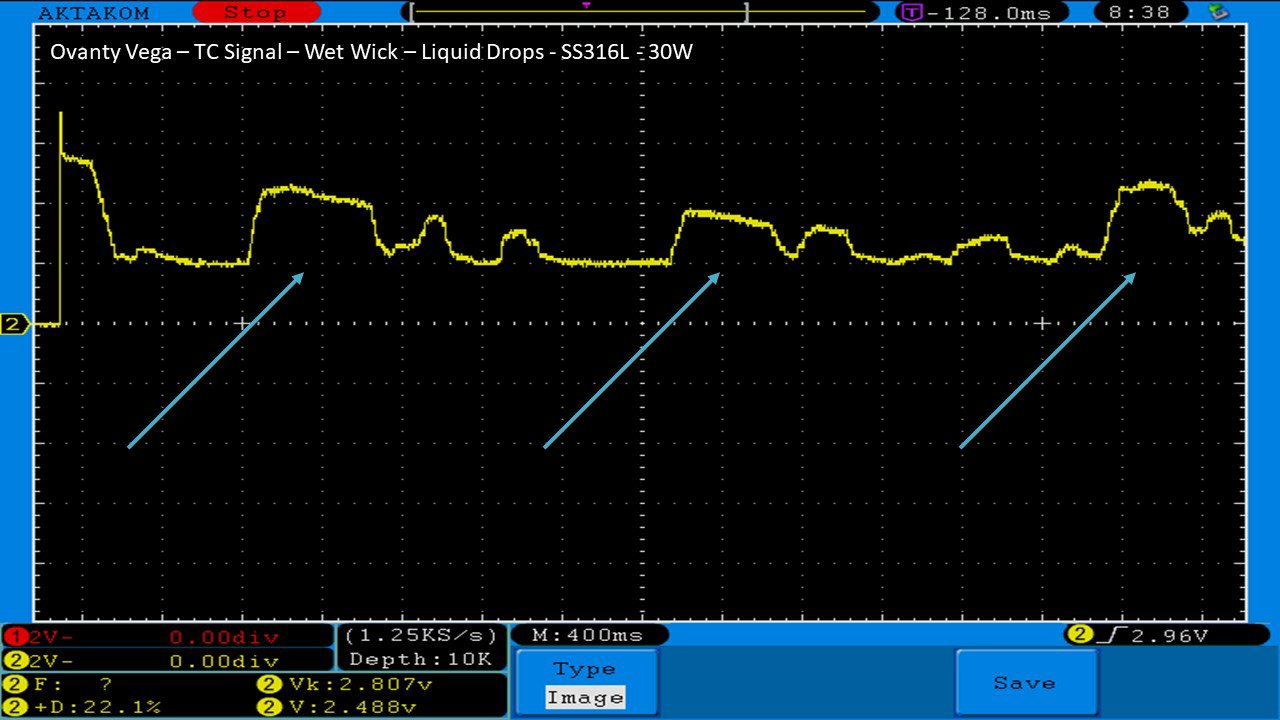
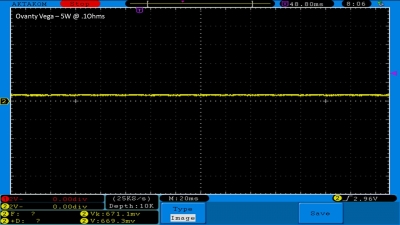
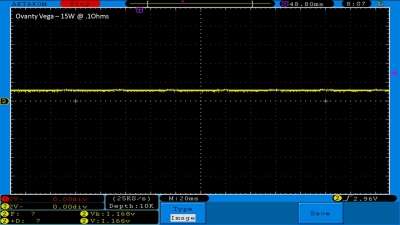
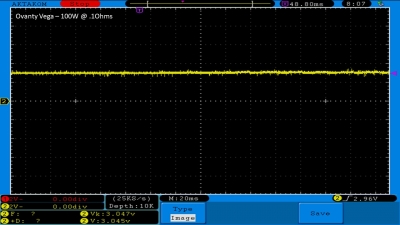
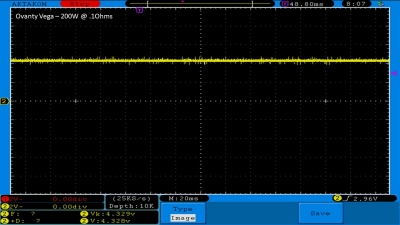
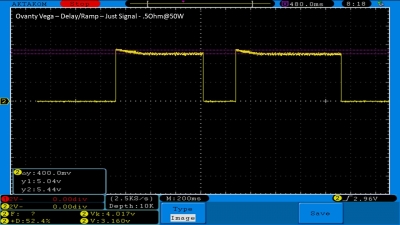
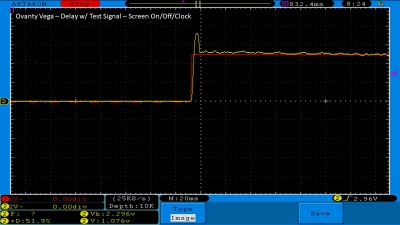
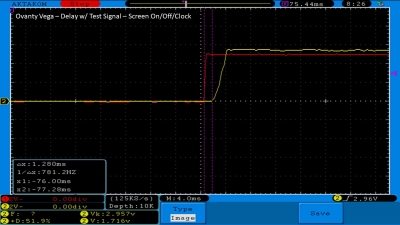
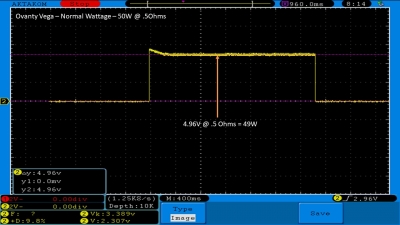
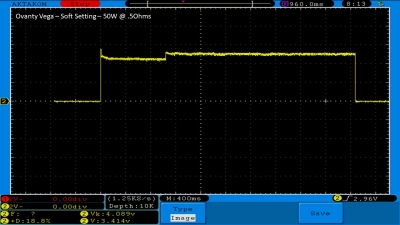
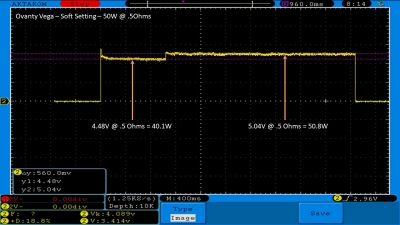
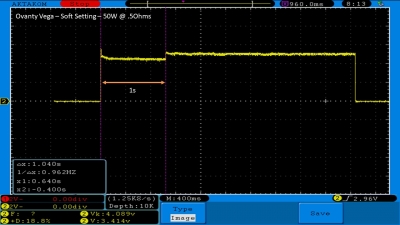
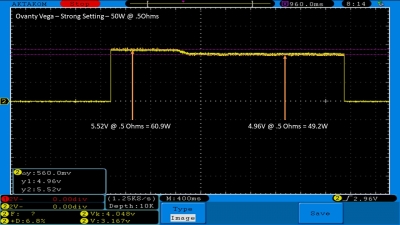
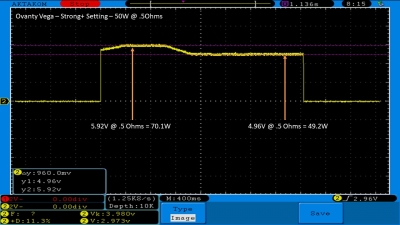
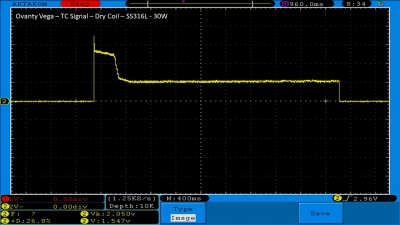
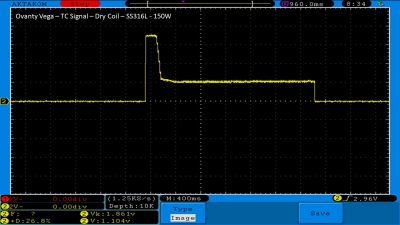
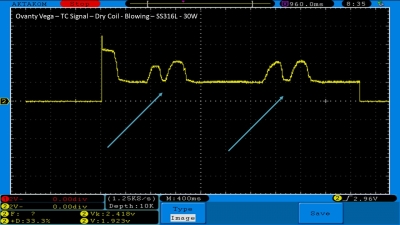
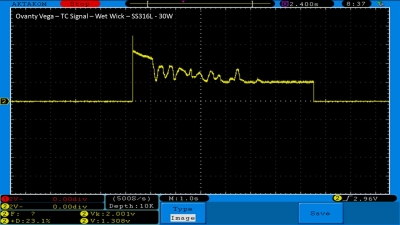
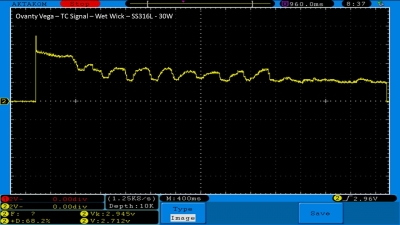
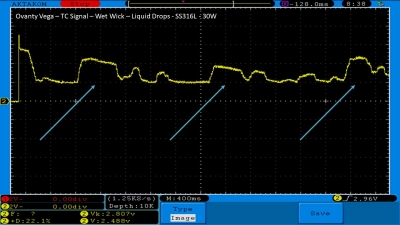
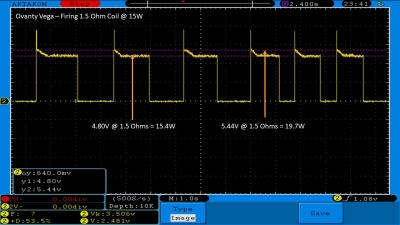
We then take a full look at the Ovanty Vega… a 200W, dual 18650 battery device.
The Links:
Paradigm Distro (Vapor)
Free Range Vapor
Kalamazoo Vapor Shop (Chef’s Recipe)
Vape Radio
Ovanty
VapeShop.sale
GeekVape
Innokin
The Video:
*NOTE: Any use of these videos in part or in their entirety without Phil Busardo’s expressed written consent is strictly prohibited.
The Photos:
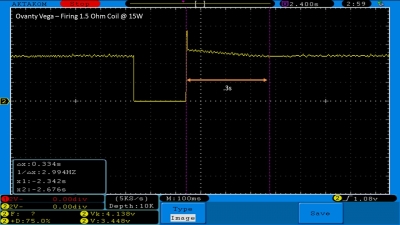
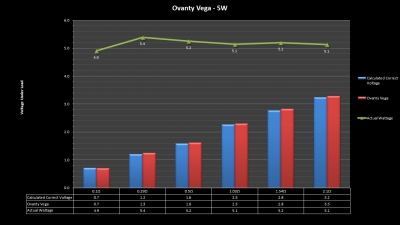
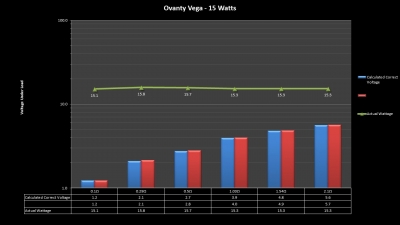
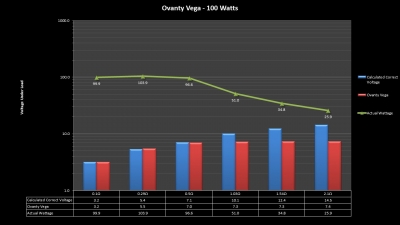
The Test Sheet:
Ovanty Vega - ReviewForm
NEW FROM REGULATOR WATCH – Vape It | It’s So Much ‘Safer’, Says Public Health Policy Strategist
Here’s the latest from Brent Stafford at Regulator Watch:
All hope is not lost when it comes to public health and its perception of vaping in Canada.
In the final installment of our “Forgotten Crisis” series—shot on location at the B.C. Centre for Disease Control. Public health consultant Penelope Hutchinson shares her transformative story from vaping skeptic to vaping advocate. And, how vaping doesn’t have to be “zero safe” in order to be “so much safer” than smoking.
It’s a remarkable interview: must watch and share—only on RegWatch by RegulatorWatch.com.
Produced by: Brent Stafford
Released May 22, 2018






























































 Store
Store












