Author: pbusardo
THE SMOKER’S SHOW TONIGHT AT 9PM EST! SPECIAL GUEST FR. JACK KEARNEY
VTA Calls For Health Officials to Disclose Facts & Act Responsibly

August 29, 2019
VTA Calls For Health Officials to Disclose Facts & Act Responsibly
In light of the seriousness of reports regarding lung disease in youth, the Vapor Technology Association (VTA) strongly urges public officials to thoroughly investigate the circumstances which might have led to each reported hospitalization before making statements to the public as to whether certain products are implicated in these incidents. To date, several public health agencies have failed to provide clear information and, instead, are unnecessarily frightening consumers by failing to distinguish between e-cigarettes and non-nicotine vaporizers.
Recent reports increasingly indicate that these adverse events are linked to illicit substances such as THC and cannabis, not e-cigarettes. For example, the New Mexico Department of Health has clearly determined that products containing THC are likely responsible for the cases highlighted in New Mexico. Despite this, virtually every other public health official continues with their generalized and repeated references to “e-cigarettes.” Such inaccurate warnings will result in either (1) people continuing to use the risky products actually causing the harm about which they have not been specifically warned; or (2) many smokers using e-cigarettes becoming ‘scared’ by these reports and moving back to deadly combustible cigarettes.
VTA condemns in the strongest possible terms the sale or use of black-market products and does not endorse the manipulation or adulteration of vapor products to consume THC, THC oil, marijuana, or synthetic products like K2. E-cigarettes and other nicotine-containing vapor products are designed for the consumption of nicotine to provide adult smokers an alternative to cigarettes; they are not intended to be used to consume illicit substances.
E-cigarettes and nicotine-containing vapor products are used by millions of adults as an alternative to combustible cigarettes, and most vapor products on the market are of high-quality. Nonetheless, no person should:
misuse or alter a vapor device designed for vaping nicotine-containing products by attempting to vape anything other than an e-liquid designed to be used with that device;
use any products other than those purchased from a reputable establishment; and
use a vapor product offered to them by someone else without knowing precisely what they are consuming.
FDA has imposed regulatory requirements on nicotine-containing vapor products for more than three years, since August 8, 2016, including strict labeling and packaging restrictions that require vapor companies to, among other requirements, disclose all of the ingredients in the products sold.
In stark contrast, none of the products designed for THC, cannabis and any other non-nicotine substances are regulated by the FDA.
Importantly, major medical groups and governments have conclusively determined that vapor products are 95% safer than combustible cigarettes, and studies have shown that they are nearly twice as effective at helping adults quit smoking than traditional methods.
From Larry
Dear Mr. Phil Busardo,
I am xx xx xx, you can call me Larry. I am 48 yrs. Old. I have a lovely wife and a beautiful daughter. I’ve been Married for 14 years. A smoker for 3 decades. And I am from the Philippines. I’ve been planning to write you this letter for about A year now to share my vaping experience to you.
I was introduced to vaping by a friend on December 2017. And my first set-up was a Vgod tube mech mod kit clone. Because it was a gift I tried it and I did not liked it. So I went to a local vape shop and asked for a set for smokers. And they gave an Avocado 24mm atty and a Wismec 80W Sinuous P80, and taught me to direct lung it. Still I hated it. The atty was also a clone. Then I find myself going to YouTube.
Watch reviews and learned from there. watched Jai Haze, Mike Vapes, DJlsb, Matt from SMM, Vap’n Fagan and then I watched you with the video of the Geekvape Aegis 100W. I collected mods and atty still nothing worked for me. I am a dual user as you may say. I smoke a pack of Marlboro lights a day. Until I finally get to watched the Smoker’s Show.
Gradually I get the motivation to quit smoking and REALLY the struggle is real. I am still a dual user. However I do find myself still smoking more than vaping. Cigarettes here in the Philippines are very cheap. A pack of Marlboro lights or reds you can only buy them for 100 pesos or about 2 us dollars. Or you can buy it on sidewalks for 12 cents each.
Lately I bought a zenith tank online. I was about to use it until the online store stopped selling the Plexus coils. So I haven’t used it yet. For the fear of liking it then stopping because I have no coils to use, plus the fact that I am still looking for the flavor that I will truly stick on daily.
Innokin products are really rare here in the Philippines. Finally last week, I pre-ordered the Adept kit and the Z-coils. Then the damn thing got unlisted from that store online. But the seller said that they will deliver it when it is ready by September 20th.
I wrote this letter to you sir Phil and also Dimitris, to remind you that I too am inspired by your work and care for smokers like me. You are not like the other reviewers on YouTube who hates smokers that are possible vapers to be added to the community.
Please forgive my English. 😁 I maybe grammatically wrong sometimes.
Please do not stop to inspire and help save People’s lives thru your work together with Dimitris.
I will stop smoking. Slowly… I can make it, thru your Smokers Show. Watching it adds inspiration and morale to smokers like me.
Thanks to you sir. And may God bless you always.
Yours Truly,
Larry
From Yves
Hello Mr Busardo.
I’d just like to thank you for everything you do around vaping. First, the
intelligent informing opinions one can read on your blog, for example this :
http://www.tasteyourjuice.com/wordpress/archives/19386
And second, I’ve finally found a way to make my coils way better watching your
videos :). So, I have a juice which finally tastes very good using my material
the way you do (a simple Siren atomizer).
Go on the good job, and take care :).
Best regards,
Yves.
INNOKIN ANNOUNCES THE T18II MINI!
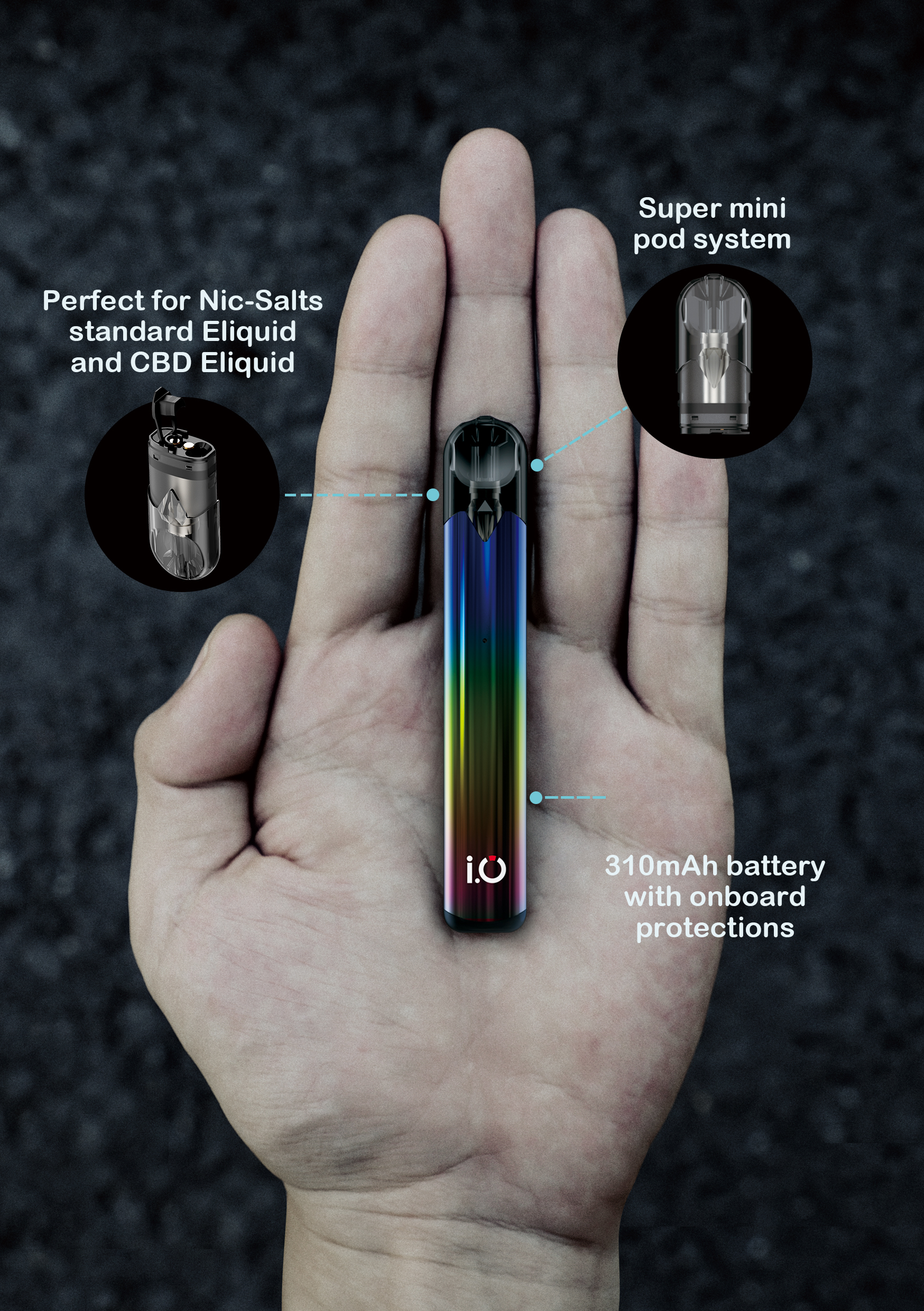
INNOKIN ANNOUNCES THE I.O – THE MIGHTY MINI VAPE
THE SMOKER’S SHOW REPLAY – S02E02 – SPECIAL GUEST DANIEL BATISTA AKA DJLSB
The Smoker’s Show S02E02 – Special Guest: Daniel Batista AKA DJlsb Vapes!
On Tonight’s show we were joined by Daniel Batista AKA DJlsb Vapes. We discuss converting smokers, the trend at vape shops, and the recent stories about people being hospitalized due to “vaping”.
As always we take your calls and answer your questions.
The Smoker’s Show is a vape show not for vapers, but for smokers.
A show to get information about vaping, to debunk vaping myths, to discuss vaping terminology & technology, and to look, review, & provide starter kits for the transitioning smoker.
We urge all vapers to invite those they know who still smoke to watch the show!
#SMOKERSWANTED
Thank you all for your support and let’s convert more smokers together!
THANK YOU TO AND PLEASE SUPPORT OUR SPONSORS!!
COUPON CODES FOR YOU…
- For 10% off Innokin/Platform Products at MyVaporStore use coupon code: MTL10
- For 15% off your Lunar Rover order use coupon code: smokersshow
THE VIDEO:
*NOTE: Any use of these videos in part or in their entirety without Phil Busardo’s and Dimitris Agrafiotis’s expressed written consent is strictly prohibited.
See everything Smoker’s Show Related HERE!
FROM THE VTA – VAPOR TECHNOLOGY ASSOCIATION AND JUUL LABS PART WAYS

APOR TECHNOLOGY ASSOCIATION AND JUUL LABS PART WAYS
AUGUST 21, 2019
Yesterday, Juul Labs announced that it would not be renewing its membership with the Vapor Technology Association (VTA) citing the lawsuit that VTA recently filed against FDA and policy differences.
VTA is surprised by Juul’s stated opposition to the filing of VTA’s complaint against FDA because Juul recently opposed the efforts of the American Academy of Pediatrics to grossly accelerate the PMTA deadline in another lawsuit and sought similar relief in that federal lawsuit. In addition, Juul’s designated VTA Board member participated in the VTA Board meeting held to consider the lawsuit against FDA, but never objected. In fact, the vote to move forward with the lawsuit was unanimous. Prior to the meeting, Juul had the relevant documents relating to VTA’s consideration of a potential lawsuit against FDA, but never objected or expressed opposition to the action.
From its inception, VTA policies have consistently focused on creating and preserving a diverse marketplace of products for adult consumers desiring an alternative to smoking cigarettes. A diverse marketplace includes both open system devices and closed system or “pod” devices. A diverse marketplace includes vape shop small business and convenience store options for adult smokers to access products. A diverse marketplace includes the many flavor options adult smokers have been purchasing in lieu of the same old tobacco and menthol flavors that are sold by Big Tobacco. A diverse marketplace includes e-liquids containing a variety of nicotine levels, including zero nicotine, to provide adult smokers with the option to reduce their nicotine intake.
We appreciate Juul’s past attempts to collaborate with VTA. We recognize that some decisions made by VTA on behalf of the industry may not perfectly align with the interests of every one of our members. However, we believe our strength as a trade association is our diverse membership.
VTA is organized so that no single member, no matter how large, can dictate policy. We put our decisions through a regimented process to make sure they are based on sound and strategic rationale. We avoid taking reactionary positions based on transitional moments or the news of the day and remain focused on securing the long-term future of a diverse and well-regulated vapor industry.
As a reminder of what is at stake, the FDA recently warned that a precipitously accelerated PMTA deadline would lead to the “mass market exit of ENDS products” and cause adults who have quit to return to smoking – a “public health outcome which should be avoided if at all possible.” VTA’s and Vapor Stockroom’s lawsuit against the FDA was filed to avoid that “mass market exit” about which FDA warned and to preserve the diverse and vibrant industry of companies dedicated to providing adult smokers an alternative to deadly cigarettes. VTA stands by its decision to defend the interests of all vapor companies, and the thousands of small businesses in the U.S. that will be put out of business without the relief requested in our lawsuit.
VTA is proud of its rapidly growing membership. We are committed more than ever to advocating for rational regulations that will ensure the availability of a diverse array of product options for adult smokers. We simply ask that all other vapor companies which believe in our vision of a diverse and vibrant marketplace join us in our fight.
Thank you for all that each of you do everyday to defend the promise of vapor!
VTA
LATEST FROM REGULATOR WATCH – Lung Scare | Respiratory Expert Questions Links to Traditional Vaping
Here’s the latest from Brent Stafford at Regulator Watch:
Progressives’ war on vaping escalated to a new and dangerous phase in the past few weeks, going well beyond mere misinformation and hysterical rhetoric.
According to the U.S. Centers for Disease Control, nearly 100 people, mostly teens, have been hospitalized with severe lung damaged; apparently the result of vaping.The allegations have made national news, yet medical officials say they do not know what products, brands or substances were being used. So how then is the CDC able to blame vaping?
In this headline edition of RegWatch we talk with Dr. Riccardo Polosa, Director of the Institute for Internal Medicine & Clinical Immunology at University of Catania, Italy. Dr. Polosa is the world’s foremost researcher studying the effects of vaping on the respiratory system, and in this episode learn why he thinks these allegations of vaping-related lung damage are beyond credible.
Only on RegWatch, by RegulatorWatch.com.
Produced by: Brent Stafford
Released: August 21, 2019**MORE ON THE LUNG SCARE – SPECIAL LIVE EDITION OF REG WATCH @ 8PM ET / 5PM PT 08.22.2019***
THE ASPIRE BREEZE NXT + CONTEST WINNERS!
A PBusardo Review – The Aspire Breeze NXT
In this video we take a look at the new Aspire Breeze NXT, the successor to the Breeze 2. I’m very impressed with this piece. We also find out who won the last 2 “Not A” Contests and kick off a new one with a chance to win your very own Aspire Breeze NXT! Enjoy!
The links:
Aspire
Element Vape
Kaees
Innokin
Eleaf
Thank you to and please support the Premiere Taste Your Juice Sponsor…
The Video:
*NOTE: Any use of these videos in part or in their entirety without Phil Busardo’s expressed written consent is strictly prohibited.
The Photos:
















































































 Store
Store












