Author: pbusardo
From Paul & Heather
Hey Phil! Just had to tell you how much I enjoy watching your videos. My
wife and I quit smoking last October. I was smoking 2 packs a day and my wife
almost 1 pack. I’m 43 and couldn’t believe how poorly I felt. We have 4 kids and
it was getting to the point Weber it seemed to be a lot of work to play and have fun
with them. Not to mention going ooze to smoke and when we travel we’d have to stop
for some breaks. My wife uncle quit smoking and started vaping with the help of
Mister E liquid. I looked into this whole vaping thing and we’ll here we are loving
every minute of it! Like I said I have learned so much from you about vaping. I
purchased a kayfun lite + and a Stingray by JD Tech that I love. I love the
flavor! My wife has a VTR with the iclear 30s and some cartos. Deadly Sin is the
bomb! Thank you so much for all you do for the vaping community. I hope to one day
shake your hand and say thanks man. P.S. Happy birthday!
Happy Vaping, Paul and Heather
TIME TO ACT!
TIME TO ACT!
CASAA has issued a press release regarding the FDA proposed rules. It has been issued through PR Newswire, posted on their blog and (if you are on their email lists) you will soon receive a copy via email.
They need our help to get this press release picked up by news outlets! (Just releasing it through a service – even a good one – doesn’t mean it will get picked up by the major news outlets.)
Here is what you can do to help:
- Forward the email to your local newspaper editors and news station producers.
- Post the press release link on the Facebook pages of local and national newspapers and networks (but please do not spam if you see it’s already been posted, just comment on the existing link that you’d like to see it reported.)
- Tweet the link to the press release to local and national newspapers and networks.
- Post a link to the press release on your Facebook timeline and in vaping groups for them to share.
For those of you chomping at the bit to “do something now,” here is your chance! There will be more calls to action as this unfolds. The email can be found below…
FOR IMMEDIATE RELEASE
WASHINGTON, April 28, 2014 /PRNewswire-USNewswire/ — Last week, the U.S. Food and Drug Administration (FDA) released its long-awaited draft regulations for electronic cigarettes (e-cigarettes) and other low-risk alternatives to smoking. The regulations offer little benefit, according to The Consumer Advocates for Smoke-free Alternatives Association (CASAA), the leading advocate for the current and future consumers of low-risk alternatives to smoking. However, CASAA believes that should the FDA finalize the rule in its current form, it will inflict devastating harm on consumers.
“This is a classic case of government imposing a ‘solution’ and then looking for a problem,” said CASAA President Julie Woessner, J.D. “The regulations do nothing to address real concerns, and instead are a slow-motion ban of the high quality e-cigarettes that have helped so many smokers quit. The rules would mostly require busy-work filings that impose huge costs with little apparent benefit.”
The proposed regulations are based on a faulty understanding of the science, reports CASAA Scientific Director, Dr. Carl V. Phillips. “FDA has cherry-picked the available evidence,” says Phillips, “blindly accepting any assertion that favors aggressive regulation and ignoring the overwhelming evidence about the harms that these regulations would cause.”
Although the regulations do not openly ban the refillable devices that are preferred by experienced users, they impose a costly registration and approval process that would effectively eliminate them. Such registrations offer minimal benefits, but ensure that only a few large companies who mass-produce small and disposable products would be able to afford the necessary filings. Additionally, while the regulations do not immediately ban the variety of popular flavors for e-cigarette liquid, they signal an intention to do so in the future.
“Our research and others’ shows that higher-quality hardware and appealing flavors are important for smoking cessation,” says Phillips. “Many former smokers report that they were always tempted to go back to smoking while using the smaller devices with imitation tobacco flavoring, but they quit smoking for good when they found better hardware and flavors that no longer reminded them of smoking.”
It is estimated that as many as a million American smokers have quit or substantially reduced their smoking thanks to e-cigarettes, and many are already making plans for a black market if these regulations take effect. Those smokers who are using e-cigarettes in a transition stage could easily return to smoking–and future potential switchers may never be able to make the transition–if the restrictions on high-quality products are imposed. Woessner, who quit smoking thanks to e-cigarettes, fears such impacts. “If I had been limited to only those products that would exist under this regulation, I would probably still be smoking.”
CASAA is preparing a response that will point out the flaws in the proposed regulations and is organizing its members and hundreds of thousand of other e-cigarette users in an attempt to persuade FDA about the harms this regulation would cause. Should that fail, it plans to fight the regulations in court.
CASAA is a 501(c)(4) nonprofit, public health, membership NGO. It does not represent the interests of industry. Donations are not tax-deductible as a charitable contribution.
Contact: Carl V Phillips, CASAA Scientific Director, 651-503-6746, cphillips@casaa.org.
PBUSARDO/TASTEYOURJUICE CONTEST RULES
Here’s a written version of the contest rules.
- Contests are contained within the video reviews. Currently running contests also appear in the upper right corner of TasteYourJuice.com, next to “Recent News”
- Contest entries should be emailed to contest@tasteyourjuice.com unless specified otherwise.
- The answer to the contest question must be contained in the subject line of the email. Nothing in the body of the email will be read.
- You must be 18+ to enter a contest.
- You must enter each contest only once. Duplicates from the same email will be removed.
- Contest winners are announced in post contest review videos.
- Contest winners have one week to respond using the same email used when entering the contest to pbusardo@tasteyourjuice.com. No response within one week forfeits your winnings and the prize will be used in a future contest. Sorry, tired of chasing you down.
- I will not respond to emails regarding contests; asking me who won, questions about the contest, if your email made it into the contest, etc. If you did things correctly, you’re entered. Note that the scroll I sometimes show in the video when picking the winner skips over MANY names and your name may not show up, even though you’re entered.
- Contest winners will be required to agree to the following:
- I am at least 18 years old.
- I will use any product won in PBusardo/TasteYourJuice contest at my own risk.
- PBusardo, Phil Busardo, or TasteYourJuice.com will not be held responsible for any damage to person or property from the use of any item won in a contest.
- PBusardo, Phil Busardo, or TasteYourJuice.com will not be responsible for repairing or replacing any item won in a contest in the event it malfunctions or arrives in a non-working state.
- Shipping outside of the US will be paid for by the contest winner.
- You will supply a photo of you with your winnings that can be used on the website.
- You understand that some items may have been opened or used. Nothing is delivered in an unsanitary state.
- You understand that some e-liquids won in a contest may have been previously opened and tested.
- You understand that e-liquids could be poisonous if used incorrectly and MUST be kept out of reach of children and pets.
CASAA Assessment of FDA Deeming Regulation, April 25, 2014
Please click their logo below to be see their assessment.
IMPORTANT:
Taking Action.
It is our current assessment that these proposed regulations are not in the best interests of consumers. They include some good provisions, but do far more harm than good. They are based on arbitrary claims and rationalizations. Should the regulations be finalized as currently formulated and implied, we are prepared to marshal our resources to file a lawsuit on behalf of consumers.
We expect to provide further analysis on Monday, April 28th, 2014. In the next week or two, we will issue a Call to Action detailing how the proposed regulations affect consumers along with suggested actions so that consumers can respond most effectively. Please remember that a comment to the FDA regulations made on Day 1 is given no more weight than a comment made on Day 75. We urge the vaping community and others interested in opposing regulation that discourages tobacco harm reduction to await further analysis before acting. There is no benefit in acting or opining precipitously.
A SUGGESTION FROM LAWYER GREG CONLEY, A FORMER CASAA DIRECTOR…
By the time most of you read this, the FDA Center for Tobacco Products will have already released the several hundred page “deeming” regulation regarding electronic cigarettes and other nicotine and tobacco products.Please read this comment I quickly wrote earlier tonight on why vapers and vendors should OPPOSE deeming regulation. I have reproduced it below this e-mail.
Predictably, the news stories out right now are largely being told from the FDA’s point of view. The FDA is trying to sell this regulation to lawmakers, public health groups, and industry, so they are of course aiming to have their regulation portrayed in the most positive light possible in the media.Nonetheless, from what I can gather from these news articles and my knowledge of the Tobacco Control Act, the deeming regulation will be a disaster for e-cigarette product innovation, small and medium-sized businesses, and consumers. This is not a surprise to me, as people and groups like Bill Godshall, Dr. Michael Siegel, CASAA, SFATA, myself, etc. have long-noted that regulation of e-cigarettes under the Tobacco Control Act would likely do more harm than good, increase costs to consumers without benefits, and lead to products being removed from the market.Please do not accept interview requests by journalists and TV reporters on this subject until you fully understand the ramifications of these regulations. Simply tell the reporters that, like any rational business owner, you won’t be commenting until you have had the chance to actually read the proposed regulations.
For business owners and investors wishing to understand the subject more, I offer consulting services. Please call or email me for more details.Best,
Gregory Conley, JD, MBA231 Church Road
Medford, NJ 08055
Here is the comment from Greg on Reddit. I thought it important enough to re-post…
Two years after these regulations go into effect, any e-cigarette product then on the market containing nicotine or any e-cigarette product marketed to be used with nicotine will be BANNED if the manufacturer does not submit a costly application to the FDA. New products will not be permitted to enter the market without FDA approval. FDA is woefully and inadequately prepared to handle this.
This is NOT good news and vendors and vapers should vehemently OPPOSE these regulations, as they will only serve to benefit large tobacco and e-cigarette companies with Wall Street investors, none of whom make the products used by 98% of this subreddit’s users. At the same time, this will potentially shut down hundreds or thousands of small and medium-sized businesses thanks to an extremely expensive, resource-heavy, and arbitrary system setup by the Family Smoking Prevention & Tobacco Control Act.
This is actually worse than I expected.
Two years after the regulation is written, e-cigarette companies will have to put in ‘new tobacco product’ applications for any product released to the market after February 25, 2007.*** This is not mere registration. This is a lengthy and expensive process. If you don’t file an application, your product is banned. If you file an application and the FDA finds that your product shouldn’t be on the market for one of a variety of reasons (including their favorite, ‘You failed to submit adequate evidence of x, y, and z.’), it can be pulled from the market.
After that 2 year date, any new e-cigarette product must be approved by the FDA before it goes to market. If the FDA does not approve your product, it cannot be brought to market. This is not a fast process, as evidenced by a Government Accountability Office report that was highly critical of huge delays that were and still are happening at the FDA Center for Tobacco Products. http://www.gao.gov/assets/660/657451.pdf
For just a sample of what a “new tobacco product” application is like, see here. This a document from when the FDA refused to even file (let alone approve) four “new tobacco product” applications : http://www.fda.gov/downloads/TobaccoProducts/Labeling/MarketingandAdvertising/UCM389515.pdf
Also see this snippet from a Lancet article (behind a paywall) by Dr. Lawrence Deyton, the former Director of the Center for Tobacco Products, in which the burden that will be put on e-cigarette and e-liquid companies is outlined: http://pastebin.com/5nGxZYac
This is bad news for e-cigarette consumers. The chance of a flavor ban or an online sales ban from the start was never really an option. This prospect was here all along and is not positive.
*** There is also something called “substantial equivalence” which is a smaller, but still expensive, loop to jump through. However, FDA guidance on substantial equivalence, and their past decisions on other applications, indicate that it would be a fruitless effort to prove substantial equivalence for a 2016 e-cig product vs. a 2007 product.
Vapers should not only oppose this deeming regulation, but also support vendors that stick up for their businesses and consumers.
A BREAKDOWN OF THE PROPOSED FDA REGULATIONS
Here is another good breakdown of the proposed FDA Regulations:
FDA Gives Huge Gift to Combustible Tobacco, and to Cancer
FDA REGULATIONS – HERE THEY ARE
Posted with permission of Dimitris and Vape Team Media.
FDA released “deeming regulations” today. Dimitris is joined by Dr. Gilbert Ross to discuss the chilling effects of these regulations and what you can do to make your voice heard.
Dr. Gilbert Ross is the Medical Director at the American Council for Science and Health.
The Video:
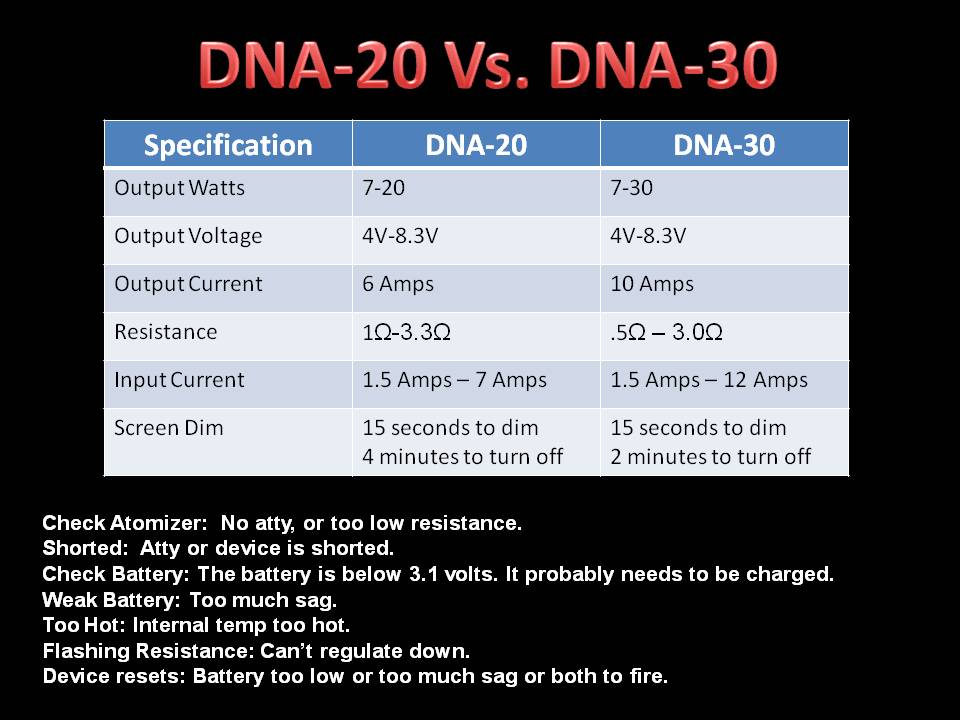
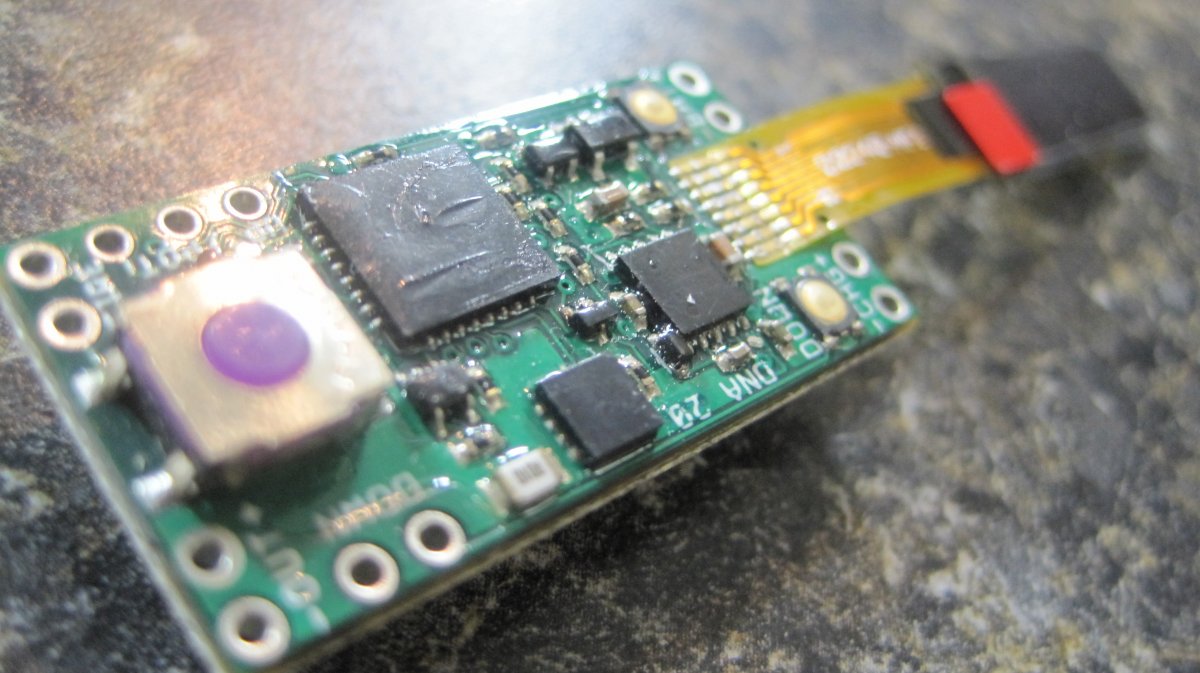
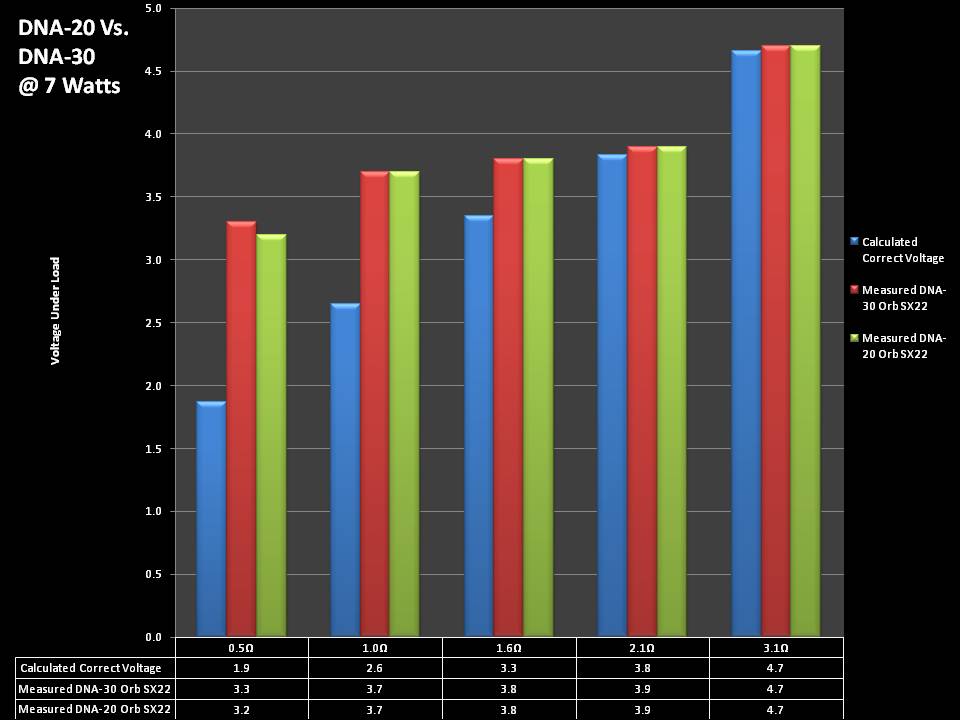
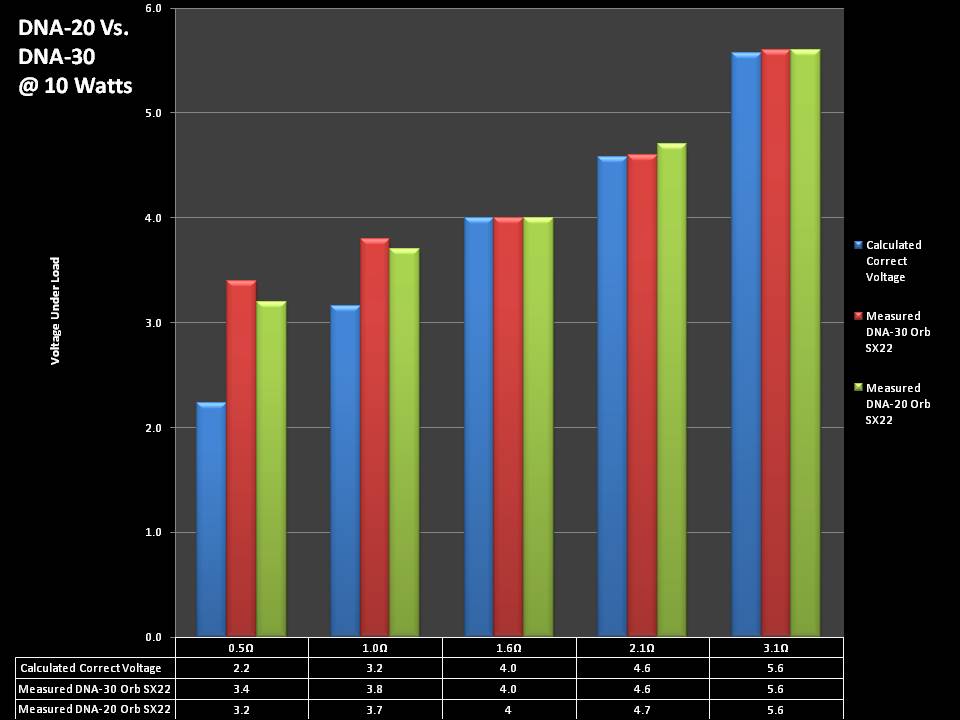
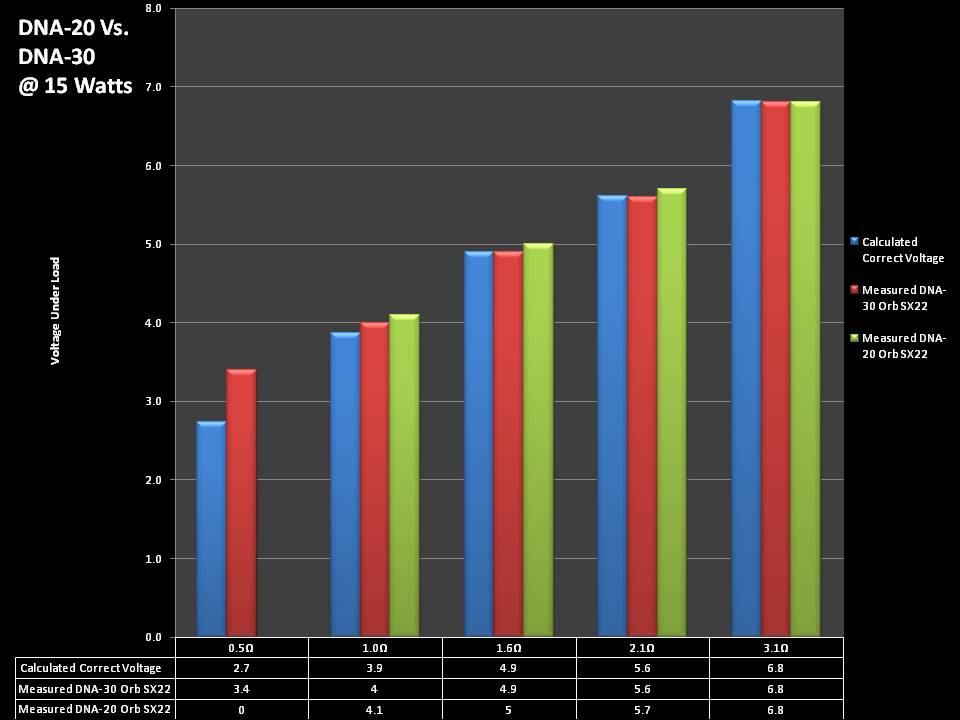
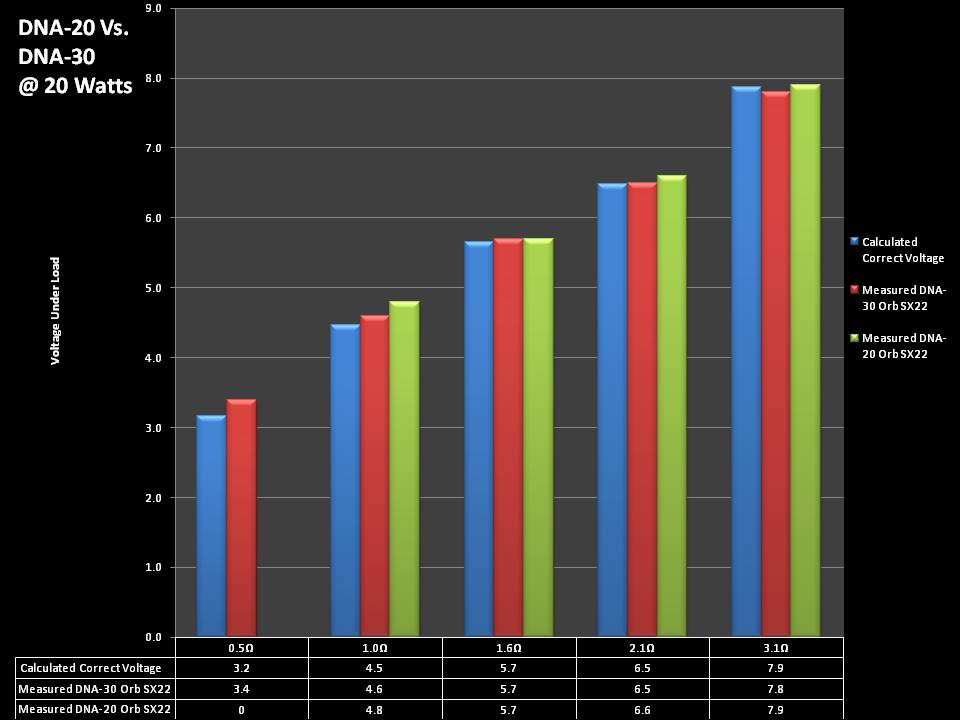
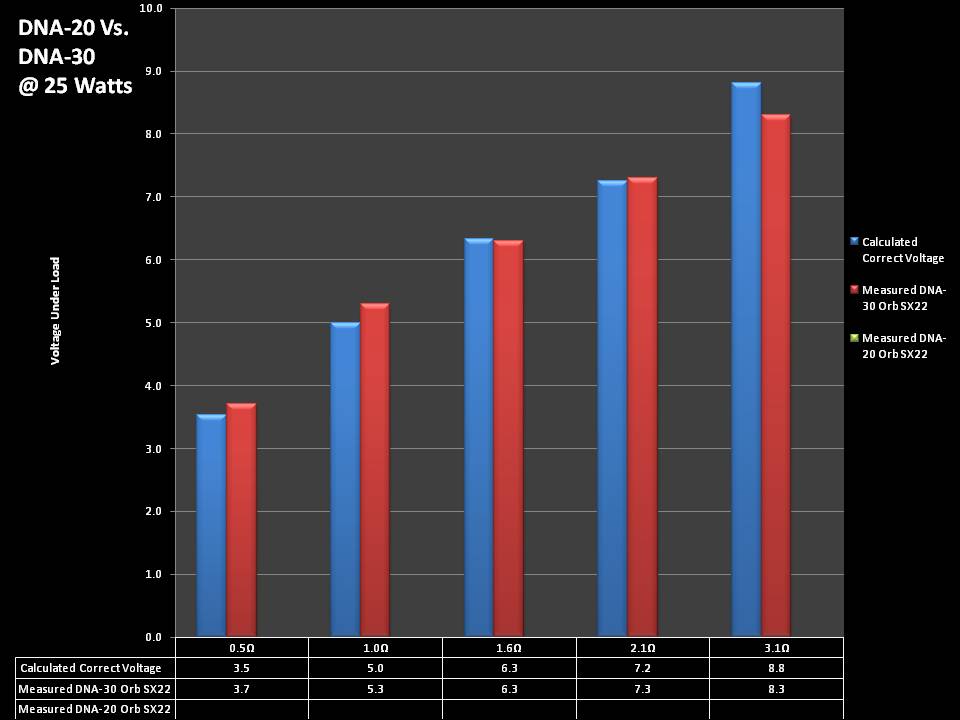
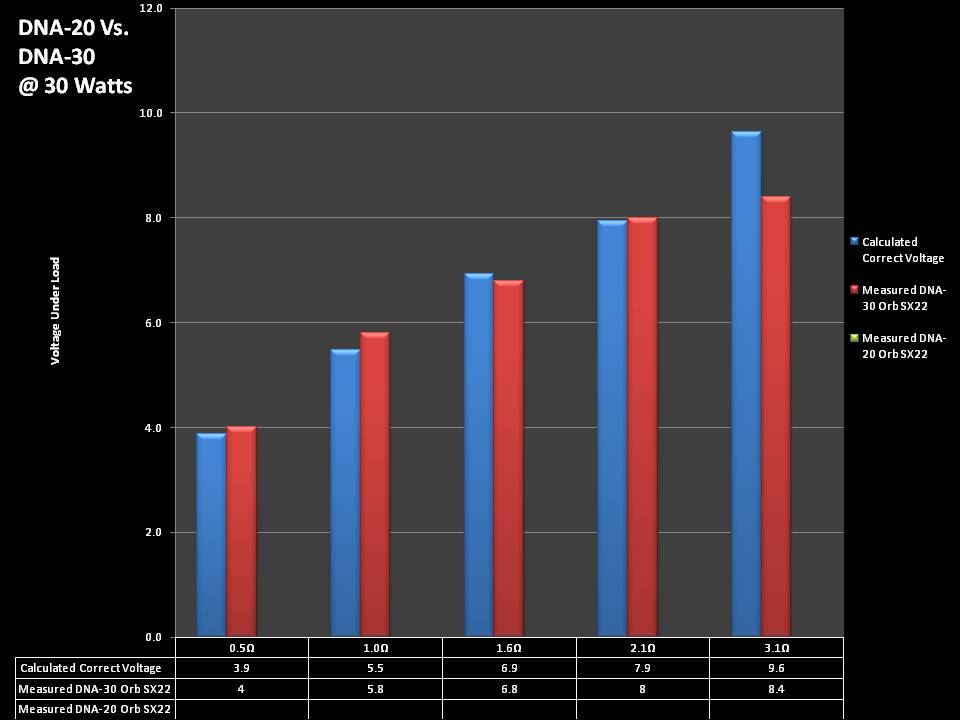
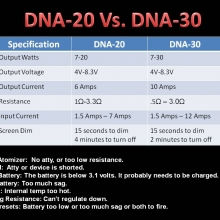
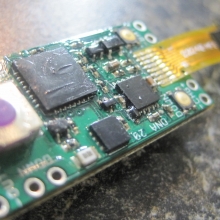
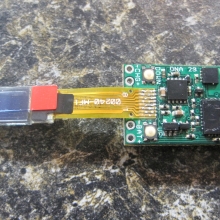
THE DNA-30 & THE CONTEST WINNERS ANNOUNCED
A PBusardo Review & Contest Winners – THE DNA 30
In this video we take a look at the DNA-30 and see how it compares to the DNA-20. We also pick the winners of the V3Tronics Flip and Seven-22 devices.
The Links:
EvolVapor – The DNA-30
V3Tronix – The Flip
Pioneer4You – The Seven-22
Vapor Ware Store – The meter
The Video:
The Photos:
Note that the DNA-20 is pictured in the photos below. The DNA-30 is the same size and format.
An interview with Jeff Stier by Dimitris
Posted with permission of Dimitris and Vape Team Media.
In this episode of It’s Political we discuss with Jeff Stier, Senior Fellow at the National Center for Public Policy Research (https://twitter.com/JeffaStier) what went down at the FDA Seminar held in San Diego April 5th as well as some interesting things stated at a press conference held at the same place! We also dive in why vapers should wake up and stand up for their right to vape!
The Video:
























 Store
Store












