Author: pbusardo
FROM CASAA – NY – Help stop senate bills that raise the age to vape and…
…ban vaping indoors!
NEW YORK ALERT
TOBACCO 21 AND INDOOR VAPING BAN
Multiple bills in New York are moving through both houses of the legislature that would ban vaping indoors and raise the age to purchase tobacco and vapor products to 21.
In the past, the NY Senate has shown more understanding and flexibility with laws affecting vaping. That being the case, we are asking members to take this opportunity to contact your state senators and urge them to oppose these misguided proposals.
Take Action – Send a Message!
Thank you,
CASAA Legislative Team
_________________________________________________
NY – S. 3978: Tobacco 21
NY – S. 2543: Indoor Vaping Ban
THE GREAT PURGE OF 2017 TO BENEFIT CASAA
It has happened. I’m at a breaking point. I need to do something about it, and this is what I’ve decided on.
Back when I started reviewing, things were a bit easier. There was a process. A manufacturer or vendor would contact me with an item to review. The item would either be approved or not. If approved, the manufacturer or vendor would be sent a questionnaire form to complete and that form had to be sent in both an email and included in the box with the product. No approval, no questionnaire, no review.
That was when things started, now I’m receiving unsolicited product on almost a daily basis. Sometimes the same product being sent by multiple manufacture’s representatives. Sometimes too many duplicates to even keep up with in the “Not A” Contests. Although this is a great problem to have, and it’s a problem I’m sincerely appreciative for, nonetheless, it’s still a problem and I’ve begun to push back.
Manufacturers and vendors must follow the standard process in order for me to look at a product either in a “Show & Tell” a “Quick Look” or a “Full Busardo” (Thanks Mark Todd 🙂 ). There will be no exceptions especially due to my limited amount of time to actually use & test products and produce the videos.
If you have interest in me looking at a product PLEASE send an email to pbusardo@tasteyourjuice.com. The email should contain a description of the product in question along with photos and links when available.
To those manufactures and vendors who DID follow the process and I will still unable to look at your products due to time constraints… thank you and my sincerest apologies.
So what do I do with all the product I’ve received and those older items in the queue?
“THE GREAT PURGE OF 2017 TO BENEFIT CASAA”
I’m going to pull a bunch of product that I still have interest in and intentions on doing videos with them and items that will wind up in the End Of Year Stocking Contest. The rest will wind up in a pile. The pile will be great. Like “open your own vape shop” great or “convert a ton of smokers great”. It will contain both old and new devices, tanks, drippers, coils, etc. You will have the opportunity to bid on the pile. Anyone is welcome to bid as long as the shipping address is in the United States… individuals, vape shops, manufacturers, organizations, etc.
The winner receives the pile and all proceeds for the auction will be donated to CASAA. Additional winner’s goodies will be announced.
I will handle all the shipping costs.
The review queue will be wiped clean.
So in the next several days as I work on the Germany video, I’m also going to be sorting through lots of product.
Stay tuned for more information on how to place your bid.
And so the pile begins…

Thanks everyone and Vape Happy My Friends!!
WHAT’S WRONG WITH E-CIGARETTES?
Simple, concise, to the point, and worth sharing. Very well done!!
May 8, 2017 Presented by CAROLINE KITCHENS
Are e-cigarettes a safe alternative to cigarettes? Could they help millions of smokers quit smoking? If so, why would anti-tobacco activists oppose e-cigarettes? Get the truth about e-cigarettes in this short video.
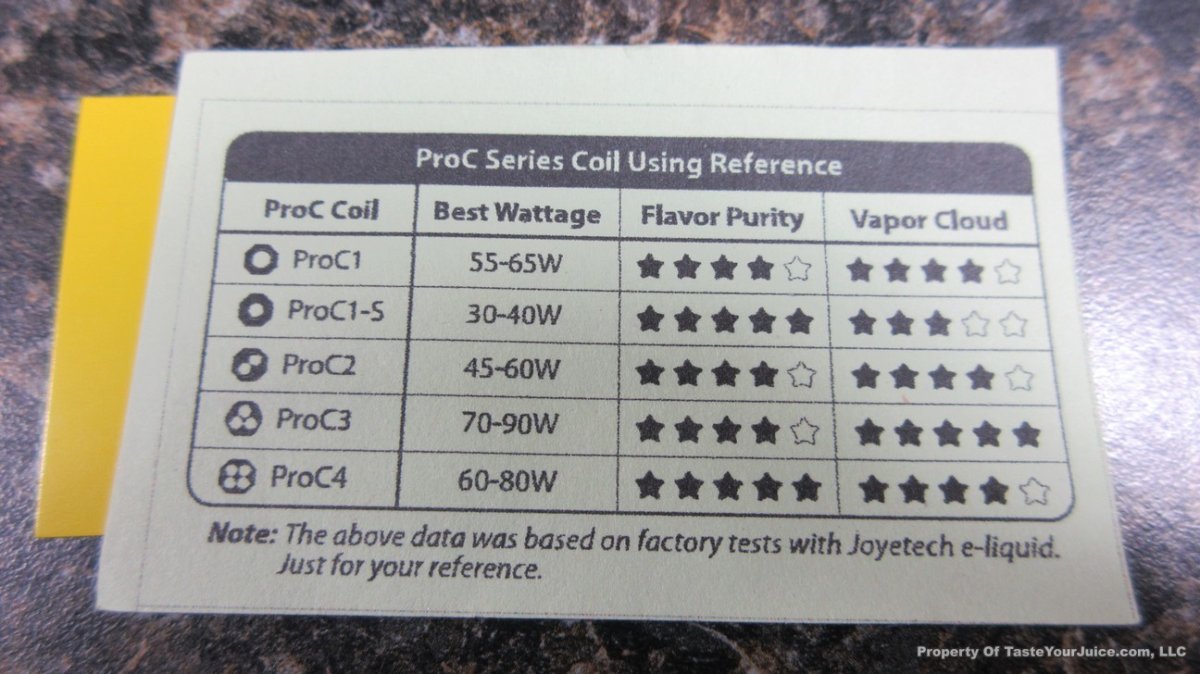
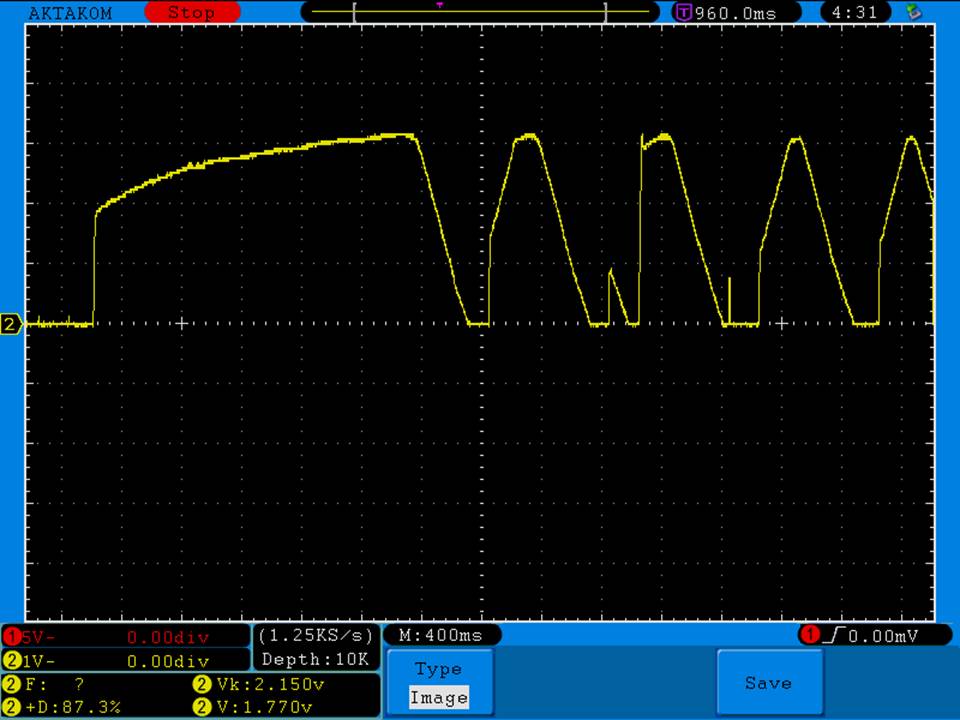
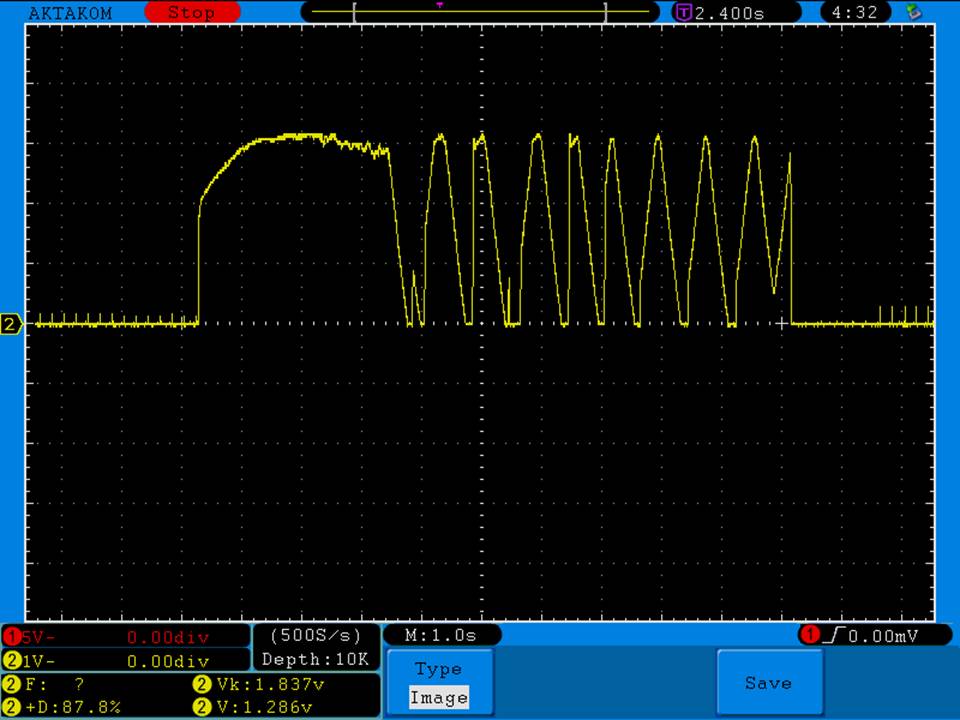
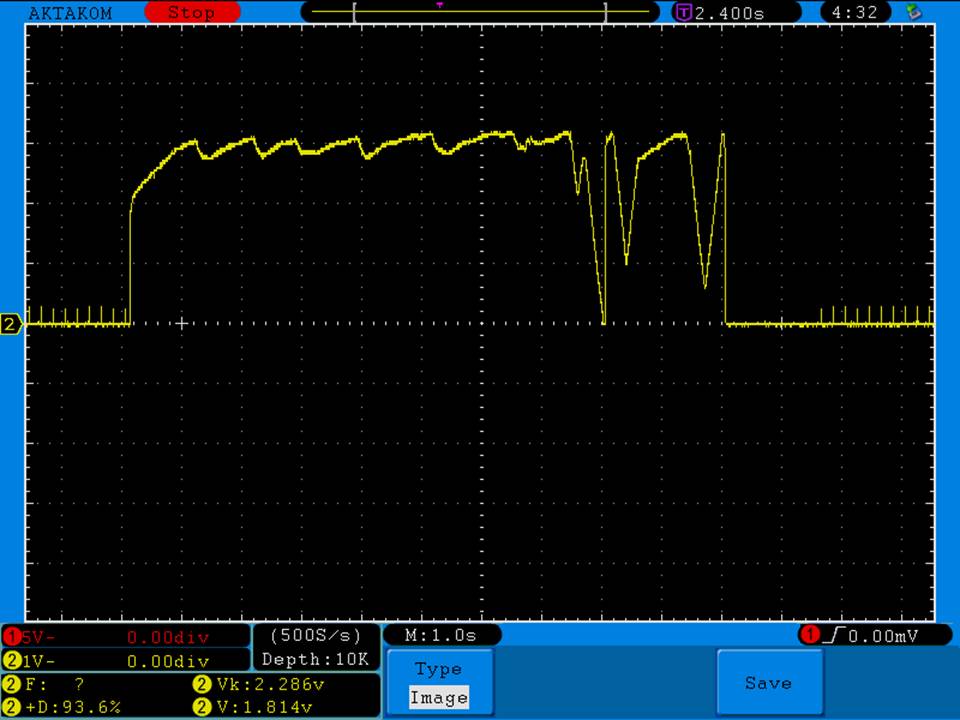
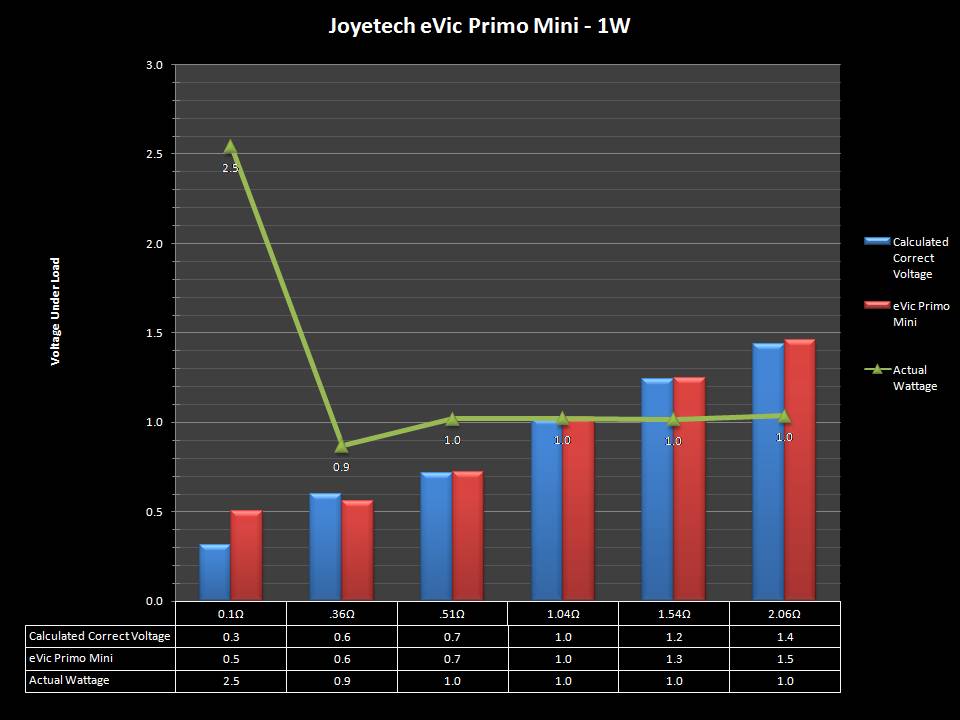
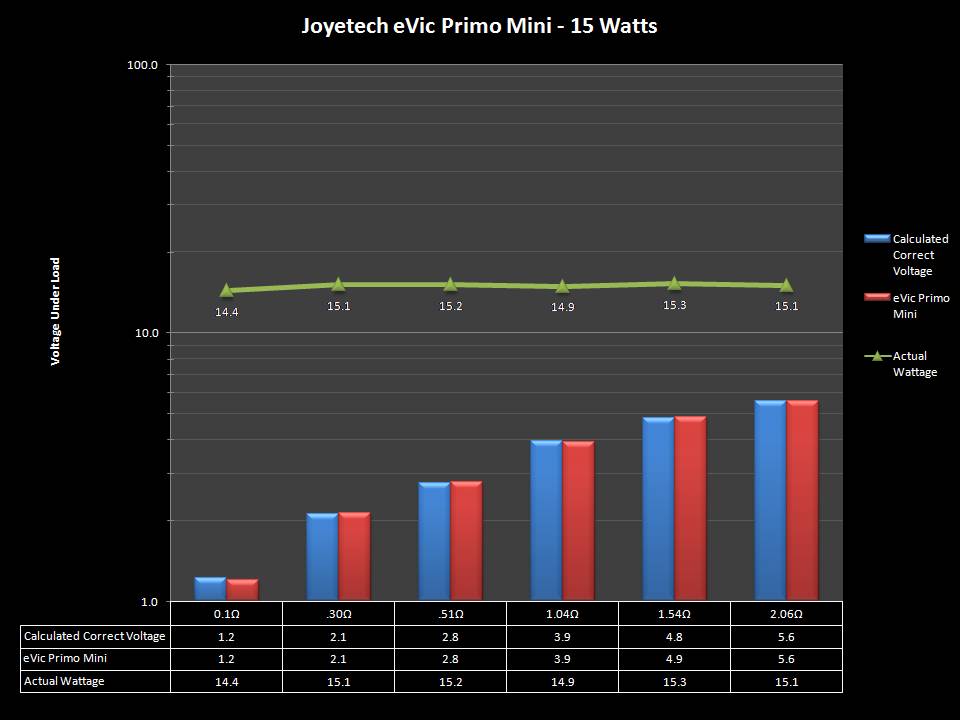
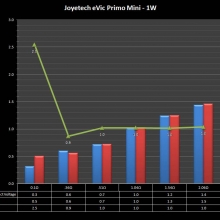
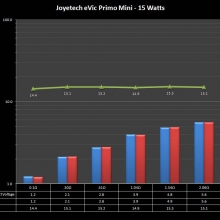
THE JOYETECH EVIC PRIMO MINI & NEW “NOT A” CONTEST
A PBusardo Review – The Joyetech eVic Primo Mini & New “Not A” Contest
In this video talk quickly about the iJust and Penguin from a MTL perspective, then take a full look at the JoyeTech eVic Primo Mini. We also touch on their new tank, the ProCore Aries & kick off a new “Not A” Contest.
The Links:
Joyetech
Eleaf
MyVaporStore
Conviction E-Liquids
Courage E-Liquids
The Video:
*NOTE: Any use of these videos in part or in their entirety without Phil Busardo’s expressed written consent is strictly prohibited.
The Photos:
FROM TONY ABBOUD/VTA – Cole-Bishop Nearly Misses; Sign Up for Federal Strategy Call


COLE-BISHOP UPDATE
On April 30, 2017, at about 9:00 p.m. eastern, House and Senate leaders reached a deal to fund the government for the next five months. Though the bill averted a government shutdown, it did not include the Cole-Bishop Amendment, which has been the vapor industry’s top legislative priority. Our intelligence tells us that while Cole-Bishop was not included, this first major attempt to incorporate bipartisan legislation in support of the industry in a major budget bill was hugely impactful. In fact, unlike previous efforts, the ultimate decision came down to just a few senators.
While we are exceptionally grateful for each of those Members of Congress who spoke up for small businesses and the vapor industry, we are particularly grateful for each vapor company, large and small, who aggressively pushed the agenda and who encouraged others to write and call their elected officials.
And, while this particular outcome is disappointing, our resolve to save vapor is strong because we have only set the stage for future success. The current budget deal runs for only 5 months. During that time, we will continue to press for a Congressional solution.
As you know, the multi-pronged plan we laid out at the beginning of 2017 included both Congressional and Administration activities. In that regard, VTA already has commenced its strategies to seek relief from the Trump Administration. Just last week, VTA leadership met with Secretary Price’s senior leadership team to make the case for that relief. In the coming months, we will be moving forward on dual paths in Congress and the Administration.
![]()
This Tuesday, May 2, 2017, at 2:00 pm (eastern), Ashley Davis and Malloy McDaniel from West Front Strategies, two members of our bi-partisan government affairs team will join us for a national webinar to provide an update on where things stand in Washington, D.C. for the vapor industry. We highly encourage you to click here to register and get the play-by-play on what went down this week and where we are heading: Please click here to register for “Cole-Bishop Outcome and All Things Federal!”
Please take a moment and forward this email to anyone you know will be interested in what’s going on and encourage them to sign up for our mailing list at www.vaportechnology.org or www.SaveVapor.org.
And, don’t forget to follow us on Facebook and Twitter.
Thank you for all you do to defend our industry!

Tony Abboud
Executive Director
Vapor Technology Association
VILLAGE OF HARTLAND FINDINGS ARE IN – CALLS THE FDA REGULATIONS “GALACTICALLY STUPID”
I’m going to be adding to this post as I get information including a summary of the effort, a complete list of all involved and links with additional information and how you can get involved and support it. So keep an eye on this post as it updates.
Thank you to all involved who work and testify on our and the industry’s behalf.
From Lou Ritter – AEMSA co-founder and first President (now emeritus), ERF Founder and President, Chairman of the ANSI TC126/SC3 TAG for vape and vapour products (Jesus Lou, that’s a lot of titles 🙂 ):
Advocacy efforts have been primarily top-down (FDA, OMB/OIRA, etc.). Various State level advocacy organizations have focused on responding to “Calls to Action” and responding at those levels. This is a different approach – sort of bottom-up as a local municipality (Village of Hartland WI) faces the impacts of the FDA regulations on their local economy, whether or not the deeming rule will close a local business and if there are justifiable health concerns to support such economic impacts – both locally and nationally.
The findings resulted in no “coordination” to fiscal impacts, no justifications, etc. This could possibly result in the FDA regulations getting a “stay” or other delay. Much of this depends on how Scott Gottlieb interprets it, his position, political pressures, and if the FDA decides to take it to court.
Below you will find all of the hearing testimony and the Findings which contains some really good news.
Unfortunately, they are not allowing the videos to be embedded here so you will need to follow the links to view them on YouTube.
Village of Hartland Public Hearing: Coordination with the FDA 4/27/2017
Hartland Public Hearing: Coordination with FDA 4/28/2017
Hartland Public Hearing: Coordination with the FDA 4/29/2017
5/1/2017 Public Hearing Board: Coordination with FDA, Findings
Vape Shop Air Sampling by California State Health Department Suggests that Secondhand Vape Exposure is Minimal
As part of its investigation into the potential health effects of electronic cigarettes, the California Department of Public Health has been conducting air sampling and personal exposure monitoring in vape shops throughout the state. The results of sampling in one of these vape shops, obtained by The Rest of the Story, reveal that “secondhand vaping” appears to result in minimal exposure of bystanders to hazardous chemicals.
This study, although conducted under very high exposure conditions in a small, non-ventilated vape shop with many employees and customers vaping and clouds of vapor visible, did not document any dangerous levels of exposure to any hazardous chemical. Nicotine exposure was essentially non-existent. Formaldehyde exposure was no different than in many indoor and outdoor environments at baseline. Acetone, acetoin, other aldehydes, toluene, benzene, and xylene were not detected. Chemicals that have been associated with “popcorn lung” were also not detected by the standard method.
Read more HERE.
E-cigarettes do not promote cancer growth in lab tests
A new study found no evidence that a commercially available e-cigarette vapor promotes the development of cancer in laboratory cells. In contrast, smoke from a reference cigarette was positive for cancer-promoting activity at very low concentrations.
Read more HERE.






























































































 Store
Store












