Category: Recent News
PBUSARDO/TASTEYOURJUICE CONTEST RULES
Here’s a written version of the contest rules.
- Contests are contained within the video reviews. Currently running contests also appear in the upper right corner of TasteYourJuice.com, next to “Recent News”
- Contest entries should be emailed to contest@tasteyourjuice.com unless specified otherwise.
- The answer to the contest question must be contained in the subject line of the email. Nothing in the body of the email will be read.
- You must be 18+ to enter a contest.
- You must enter each contest only once. Duplicates from the same email will be removed.
- Contest winners are announced in post contest review videos.
- Contest winners have one week to respond using the same email used when entering the contest to pbusardo@tasteyourjuice.com. No response within one week forfeits your winnings and the prize will be used in a future contest. Sorry, tired of chasing you down.
- I will not respond to emails regarding contests; asking me who won, questions about the contest, if your email made it into the contest, etc. If you did things correctly, you’re entered. Note that the scroll I sometimes show in the video when picking the winner skips over MANY names and your name may not show up, even though you’re entered.
- Contest winners will be required to agree to the following:
- I am at least 18 years old.
- I will use any product won in PBusardo/TasteYourJuice contest at my own risk.
- PBusardo, Phil Busardo, or TasteYourJuice.com will not be held responsible for any damage to person or property from the use of any item won in a contest.
- PBusardo, Phil Busardo, or TasteYourJuice.com will not be responsible for repairing or replacing any item won in a contest in the event it malfunctions or arrives in a non-working state.
- Shipping outside of the US will be paid for by the contest winner.
- You will supply a photo of you with your winnings that can be used on the website.
- You understand that some items may have been opened or used. Nothing is delivered in an unsanitary state.
- You understand that some e-liquids won in a contest may have been previously opened and tested.
- You understand that e-liquids could be poisonous if used incorrectly and MUST be kept out of reach of children and pets.
CASAA Assessment of FDA Deeming Regulation, April 25, 2014
Please click their logo below to be see their assessment.
IMPORTANT:
Taking Action.
It is our current assessment that these proposed regulations are not in the best interests of consumers. They include some good provisions, but do far more harm than good. They are based on arbitrary claims and rationalizations. Should the regulations be finalized as currently formulated and implied, we are prepared to marshal our resources to file a lawsuit on behalf of consumers.
We expect to provide further analysis on Monday, April 28th, 2014. In the next week or two, we will issue a Call to Action detailing how the proposed regulations affect consumers along with suggested actions so that consumers can respond most effectively. Please remember that a comment to the FDA regulations made on Day 1 is given no more weight than a comment made on Day 75. We urge the vaping community and others interested in opposing regulation that discourages tobacco harm reduction to await further analysis before acting. There is no benefit in acting or opining precipitously.
A SUGGESTION FROM LAWYER GREG CONLEY, A FORMER CASAA DIRECTOR…
By the time most of you read this, the FDA Center for Tobacco Products will have already released the several hundred page “deeming” regulation regarding electronic cigarettes and other nicotine and tobacco products.Please read this comment I quickly wrote earlier tonight on why vapers and vendors should OPPOSE deeming regulation. I have reproduced it below this e-mail.
Predictably, the news stories out right now are largely being told from the FDA’s point of view. The FDA is trying to sell this regulation to lawmakers, public health groups, and industry, so they are of course aiming to have their regulation portrayed in the most positive light possible in the media.Nonetheless, from what I can gather from these news articles and my knowledge of the Tobacco Control Act, the deeming regulation will be a disaster for e-cigarette product innovation, small and medium-sized businesses, and consumers. This is not a surprise to me, as people and groups like Bill Godshall, Dr. Michael Siegel, CASAA, SFATA, myself, etc. have long-noted that regulation of e-cigarettes under the Tobacco Control Act would likely do more harm than good, increase costs to consumers without benefits, and lead to products being removed from the market.Please do not accept interview requests by journalists and TV reporters on this subject until you fully understand the ramifications of these regulations. Simply tell the reporters that, like any rational business owner, you won’t be commenting until you have had the chance to actually read the proposed regulations.
For business owners and investors wishing to understand the subject more, I offer consulting services. Please call or email me for more details.Best,
Gregory Conley, JD, MBA231 Church Road
Medford, NJ 08055
Here is the comment from Greg on Reddit. I thought it important enough to re-post…
Two years after these regulations go into effect, any e-cigarette product then on the market containing nicotine or any e-cigarette product marketed to be used with nicotine will be BANNED if the manufacturer does not submit a costly application to the FDA. New products will not be permitted to enter the market without FDA approval. FDA is woefully and inadequately prepared to handle this.
This is NOT good news and vendors and vapers should vehemently OPPOSE these regulations, as they will only serve to benefit large tobacco and e-cigarette companies with Wall Street investors, none of whom make the products used by 98% of this subreddit’s users. At the same time, this will potentially shut down hundreds or thousands of small and medium-sized businesses thanks to an extremely expensive, resource-heavy, and arbitrary system setup by the Family Smoking Prevention & Tobacco Control Act.
This is actually worse than I expected.
Two years after the regulation is written, e-cigarette companies will have to put in ‘new tobacco product’ applications for any product released to the market after February 25, 2007.*** This is not mere registration. This is a lengthy and expensive process. If you don’t file an application, your product is banned. If you file an application and the FDA finds that your product shouldn’t be on the market for one of a variety of reasons (including their favorite, ‘You failed to submit adequate evidence of x, y, and z.’), it can be pulled from the market.
After that 2 year date, any new e-cigarette product must be approved by the FDA before it goes to market. If the FDA does not approve your product, it cannot be brought to market. This is not a fast process, as evidenced by a Government Accountability Office report that was highly critical of huge delays that were and still are happening at the FDA Center for Tobacco Products. http://www.gao.gov/assets/660/657451.pdf
For just a sample of what a “new tobacco product” application is like, see here. This a document from when the FDA refused to even file (let alone approve) four “new tobacco product” applications : http://www.fda.gov/downloads/TobaccoProducts/Labeling/MarketingandAdvertising/UCM389515.pdf
Also see this snippet from a Lancet article (behind a paywall) by Dr. Lawrence Deyton, the former Director of the Center for Tobacco Products, in which the burden that will be put on e-cigarette and e-liquid companies is outlined: http://pastebin.com/5nGxZYac
This is bad news for e-cigarette consumers. The chance of a flavor ban or an online sales ban from the start was never really an option. This prospect was here all along and is not positive.
*** There is also something called “substantial equivalence” which is a smaller, but still expensive, loop to jump through. However, FDA guidance on substantial equivalence, and their past decisions on other applications, indicate that it would be a fruitless effort to prove substantial equivalence for a 2016 e-cig product vs. a 2007 product.
Vapers should not only oppose this deeming regulation, but also support vendors that stick up for their businesses and consumers.
A BREAKDOWN OF THE PROPOSED FDA REGULATIONS
Here is another good breakdown of the proposed FDA Regulations:
FDA Gives Huge Gift to Combustible Tobacco, and to Cancer
FDA REGULATIONS – HERE THEY ARE
Posted with permission of Dimitris and Vape Team Media.
FDA released “deeming regulations” today. Dimitris is joined by Dr. Gilbert Ross to discuss the chilling effects of these regulations and what you can do to make your voice heard.
Dr. Gilbert Ross is the Medical Director at the American Council for Science and Health.
The Video:
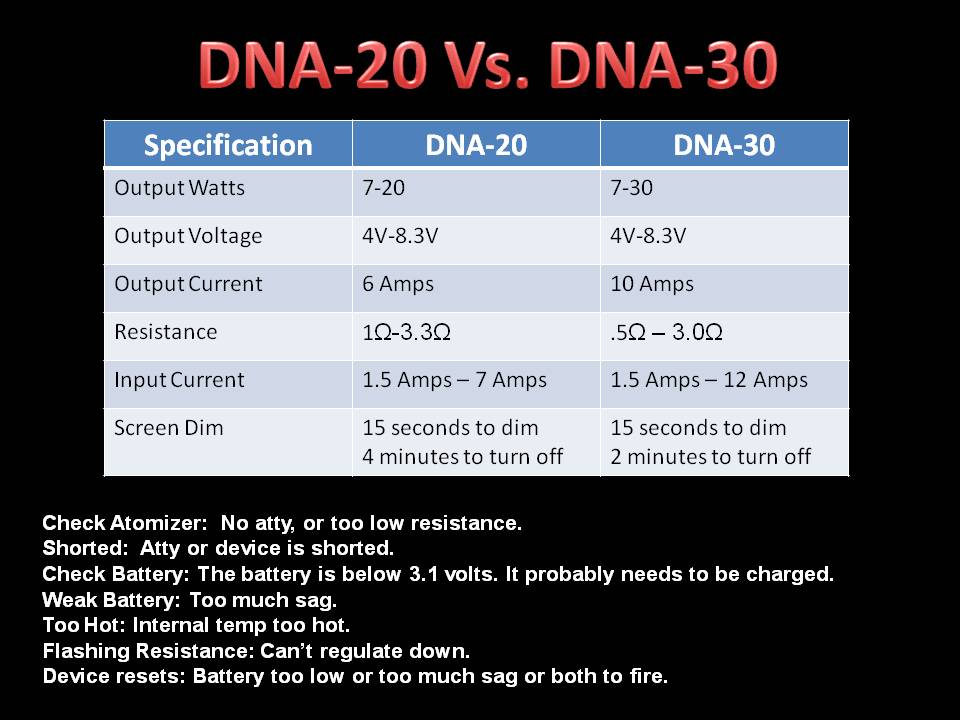
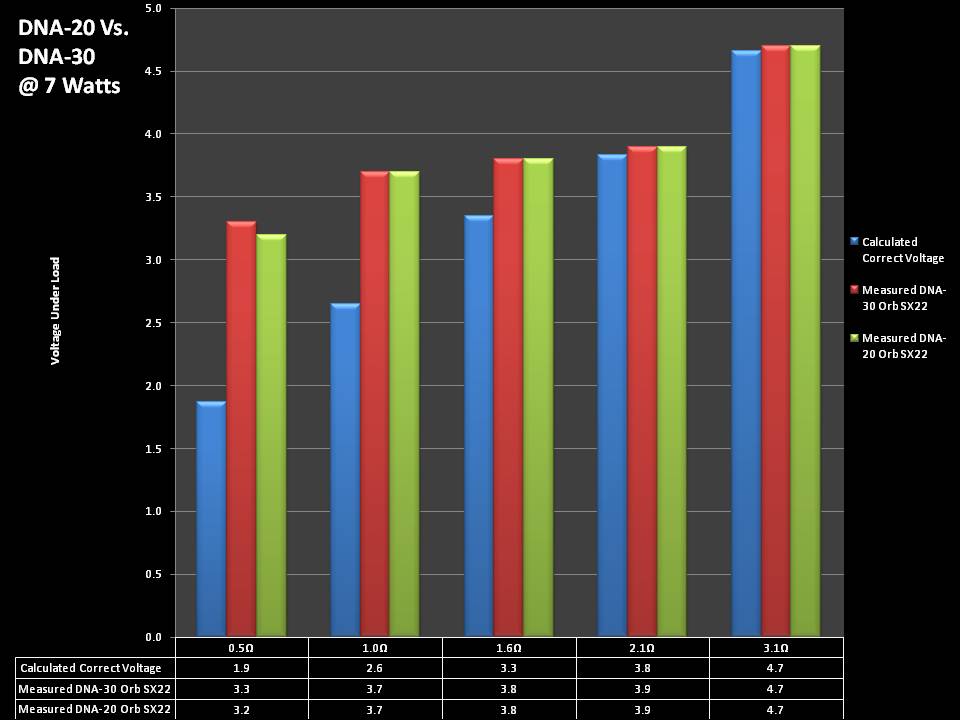
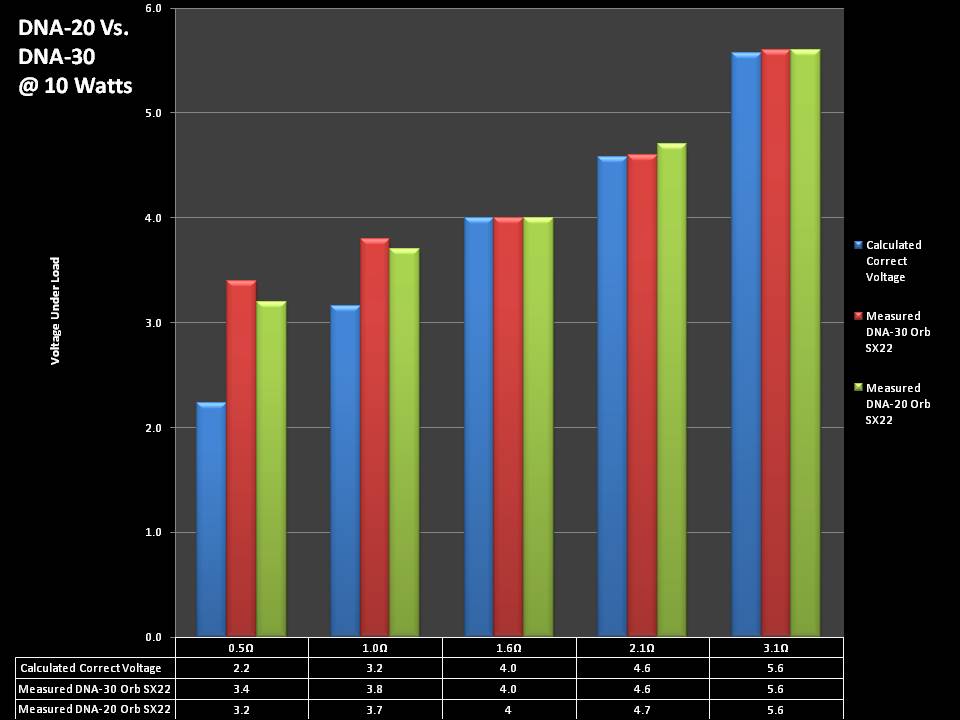
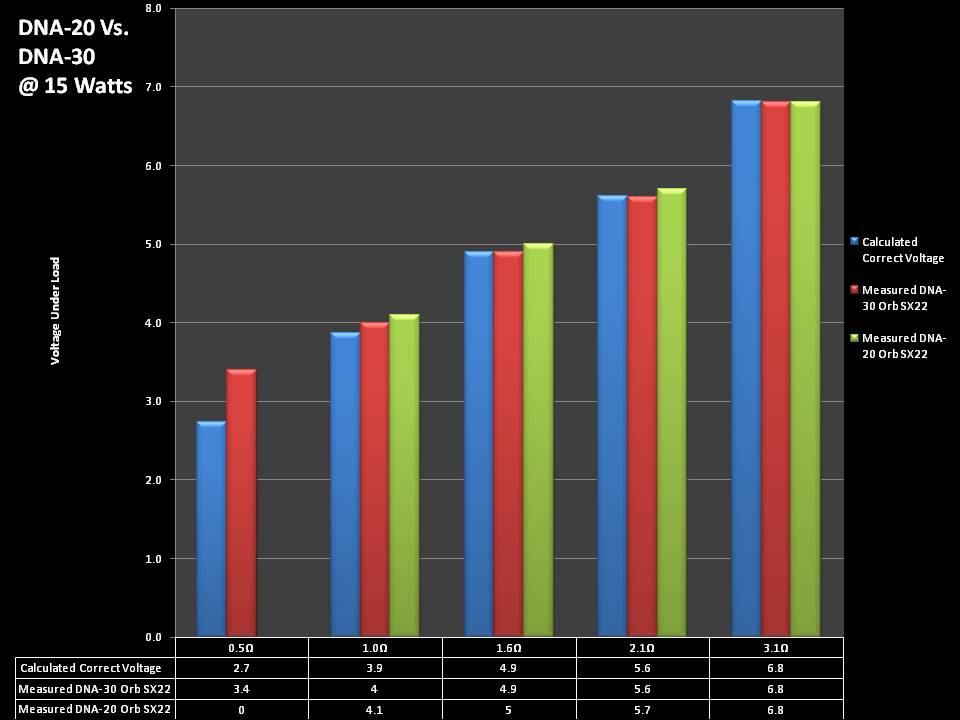
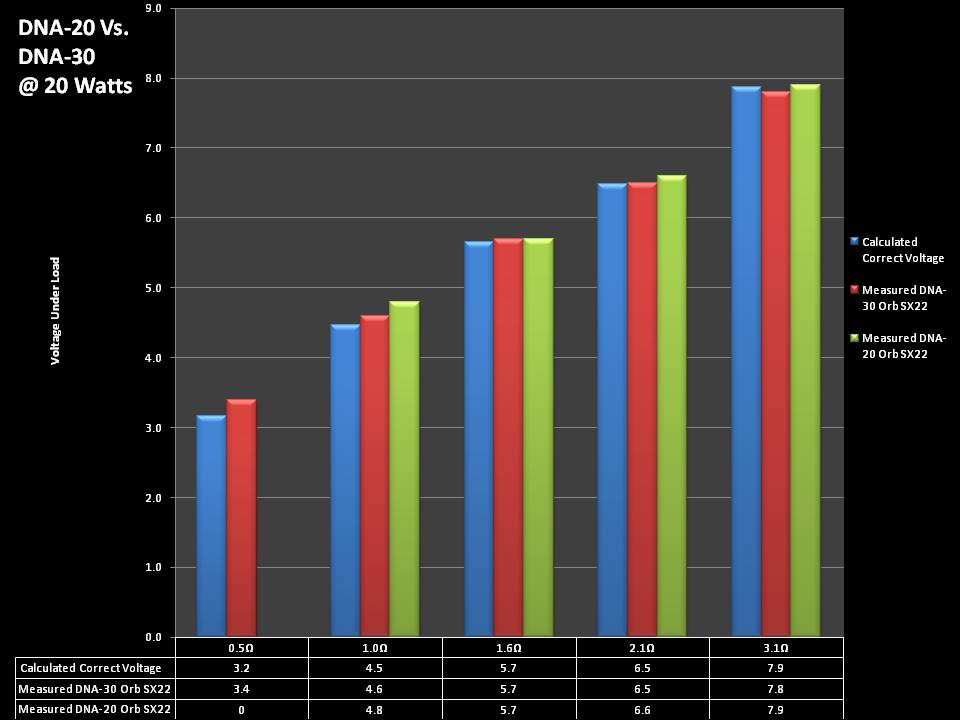
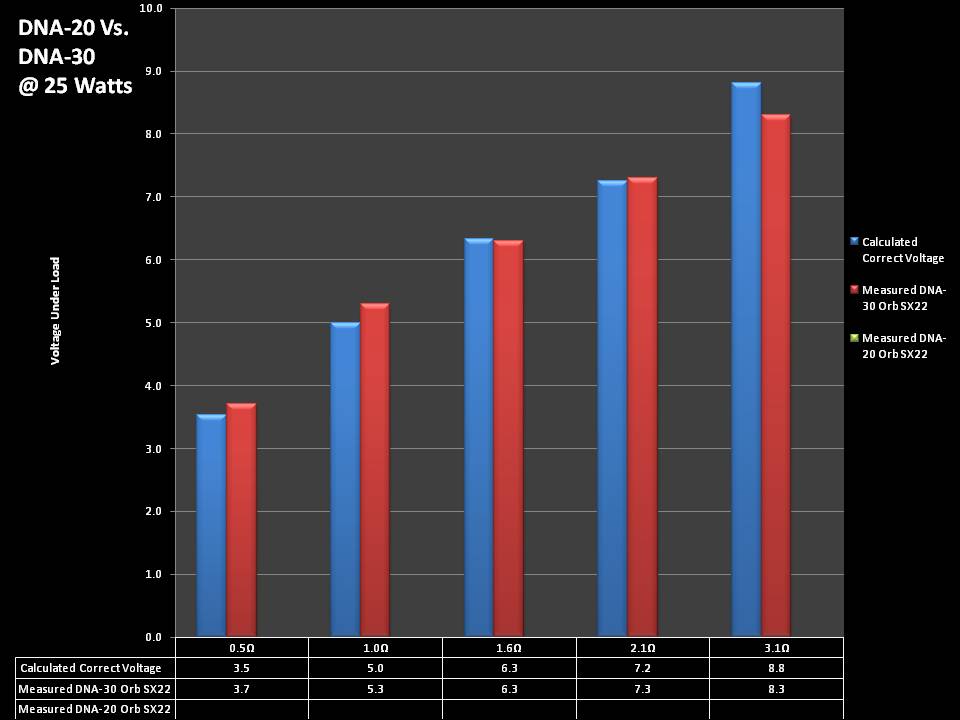
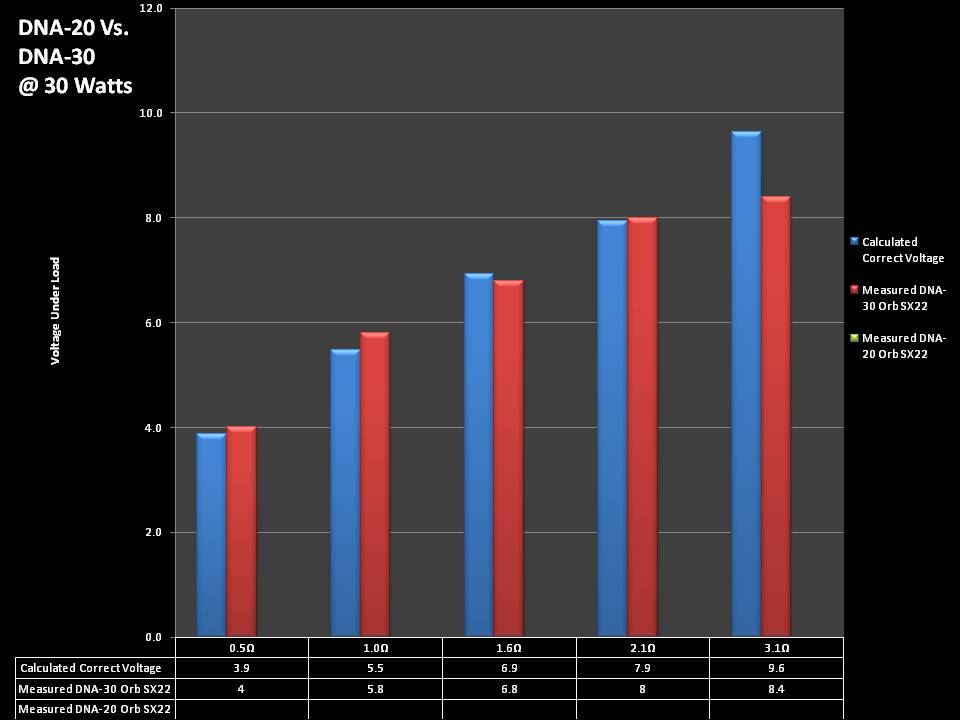
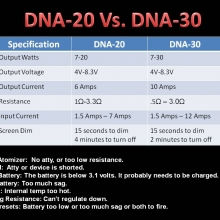
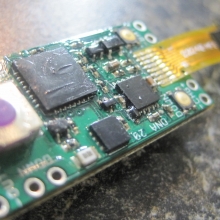
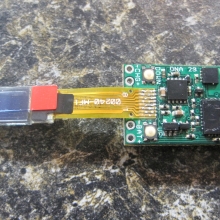
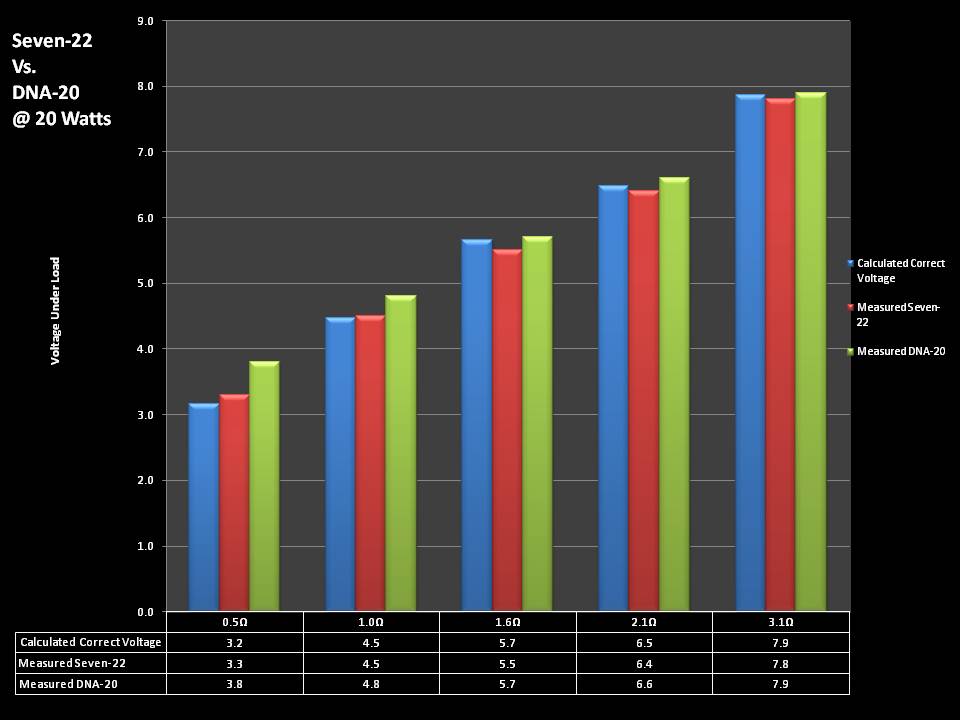
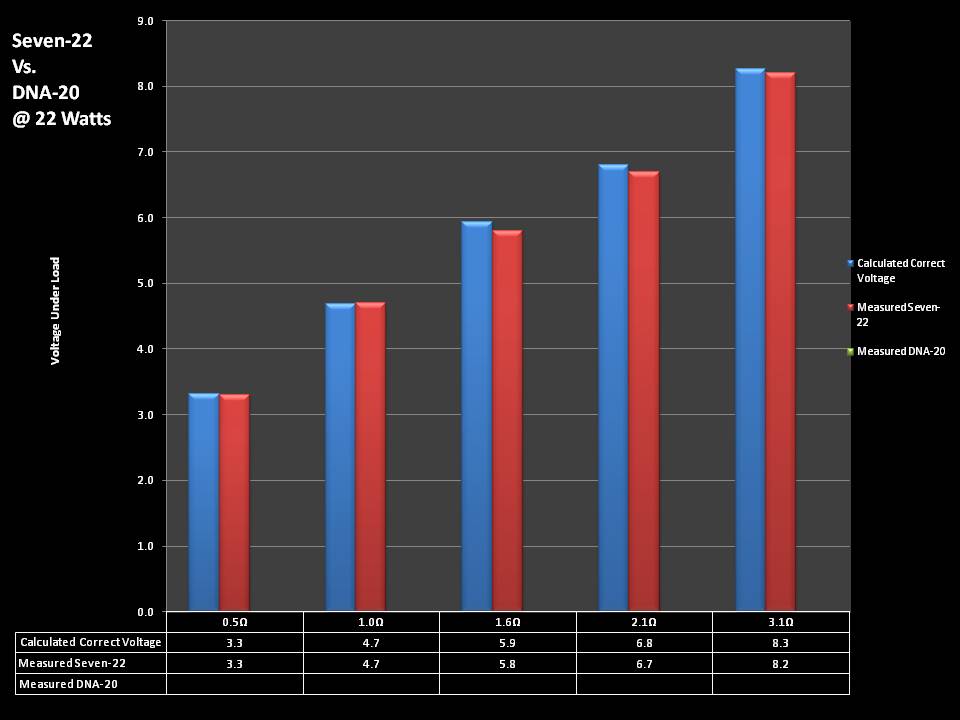
THE DNA-30 & THE CONTEST WINNERS ANNOUNCED
A PBusardo Review & Contest Winners – THE DNA 30
In this video we take a look at the DNA-30 and see how it compares to the DNA-20. We also pick the winners of the V3Tronics Flip and Seven-22 devices.
The Links:
EvolVapor – The DNA-30
V3Tronix – The Flip
Pioneer4You – The Seven-22
Vapor Ware Store – The meter
The Video:
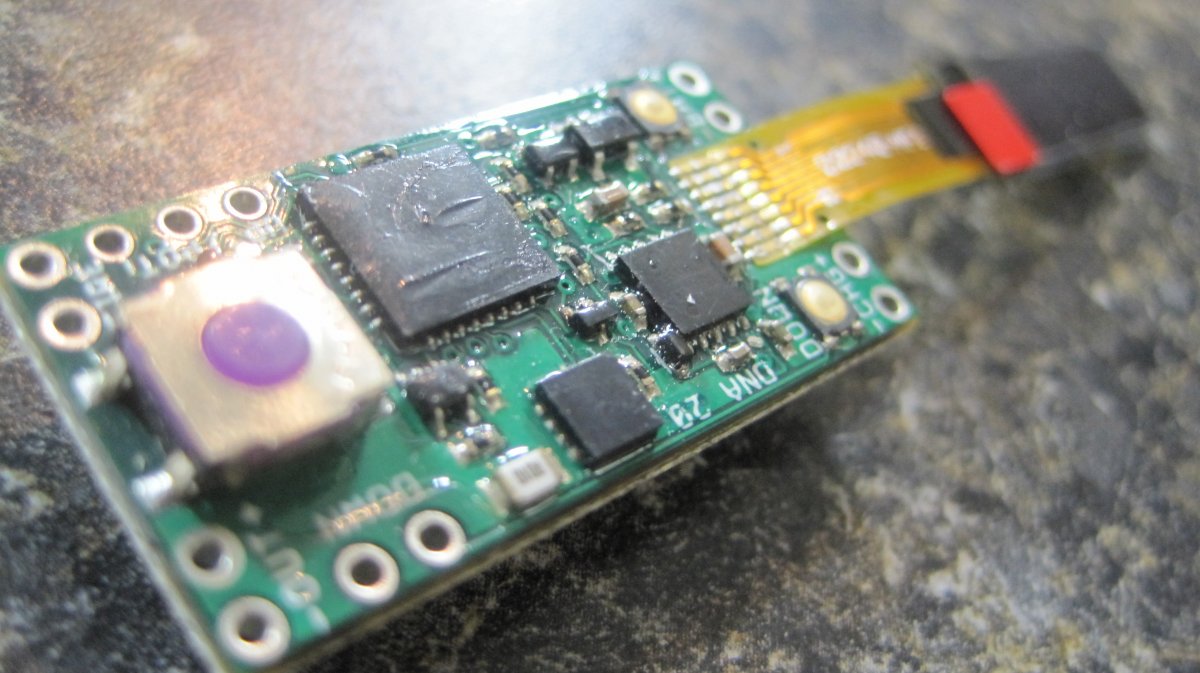
The Photos:
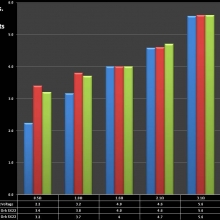
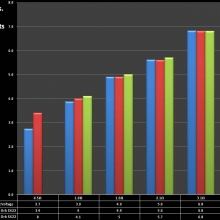
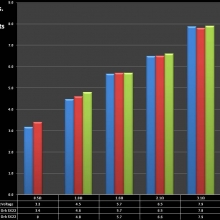
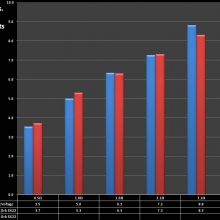
Note that the DNA-20 is pictured in the photos below. The DNA-30 is the same size and format.
An interview with Jeff Stier by Dimitris
Posted with permission of Dimitris and Vape Team Media.
In this episode of It’s Political we discuss with Jeff Stier, Senior Fellow at the National Center for Public Policy Research (https://twitter.com/JeffaStier) what went down at the FDA Seminar held in San Diego April 5th as well as some interesting things stated at a press conference held at the same place! We also dive in why vapers should wake up and stand up for their right to vape!
The Video:
NEW IN THE QUEUE – 04/19/14
With this New In The Queue update, the review queue is now closed for the summer, although a few more items may trickle in.
DON’T PANIC! 🙂
I’ll still be doing review videos over the summer, but this will allow me to catch up a bit and spend some much needed time with family and friends.
You may continue to submit items for review, but unless they are “Vape Shattering” they will probably not be accepted until after the summer is over.
Thanks for your understanding and continued support!
- The Radix V1
- The Vapor Shark DNA-30
- New items from Clean Tanks
- The Arnold RTA – Website coming soon
- The D-Eagle RTA
- The Eye APV
- The Mechano Double M Mechanical
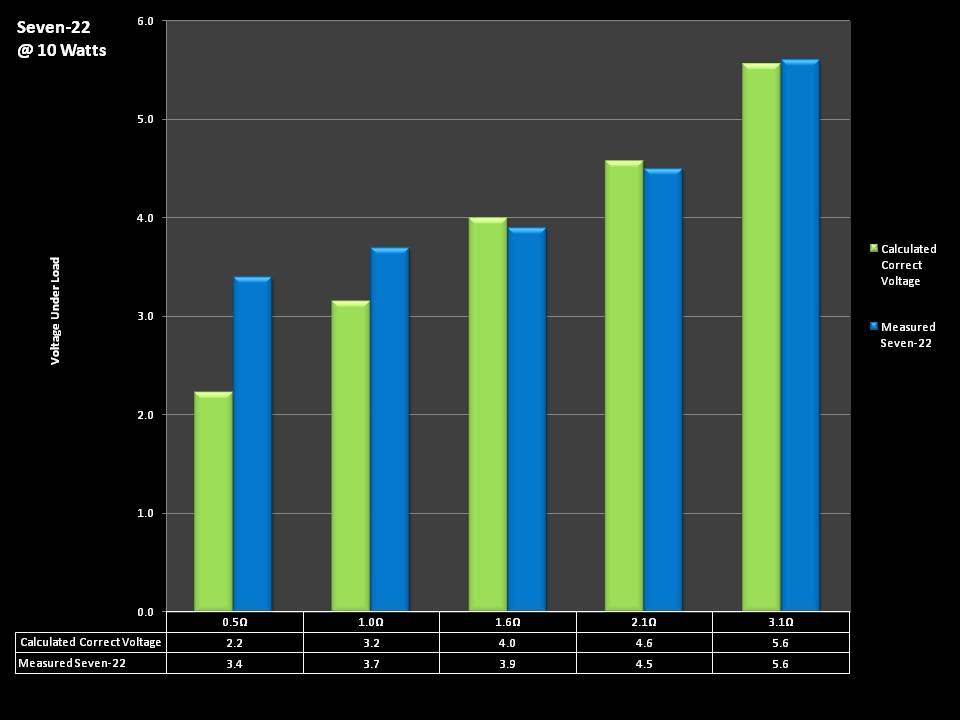
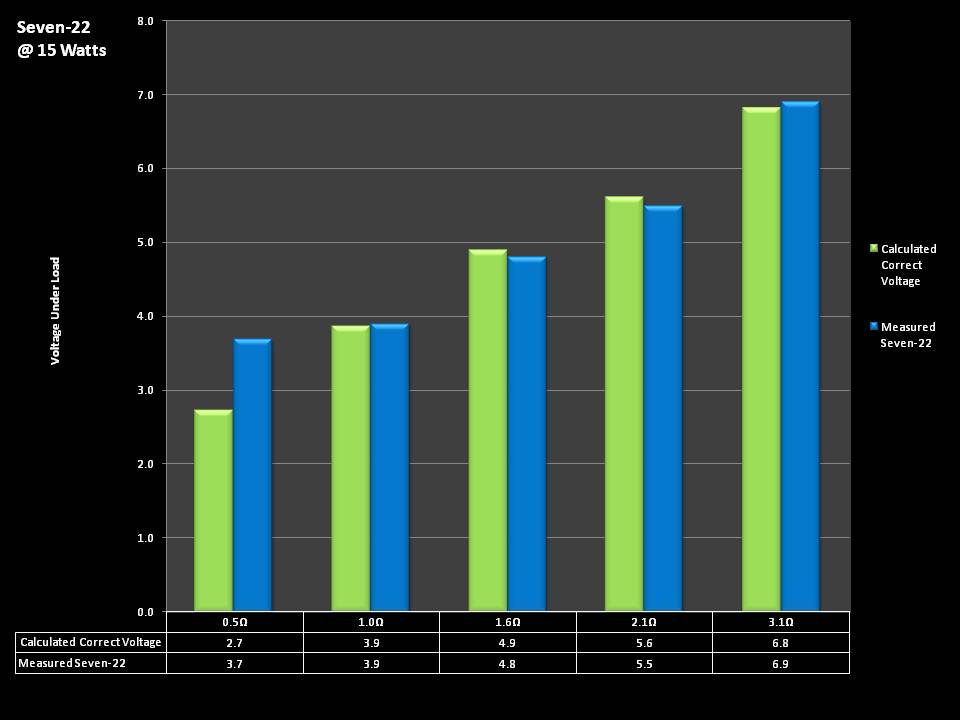
THE SEVEN-22 REVIEW & CONTEST!
A PBusardo Review & Contest – The Seven 22
In this video we take a full look at the Seven 22, we do a show & tell on the Vapor Ware resistance and voltage meter, we go over contest rules and how to enter, and we kick off a new contest for a Seven-22 and a Vaper Ware meter!
The Links:
Alibaba Product Page
Poneer4U Website
Distribution Contact Email
Vapor Ware Store – For the meter
The Video:
The Photos:















































































































































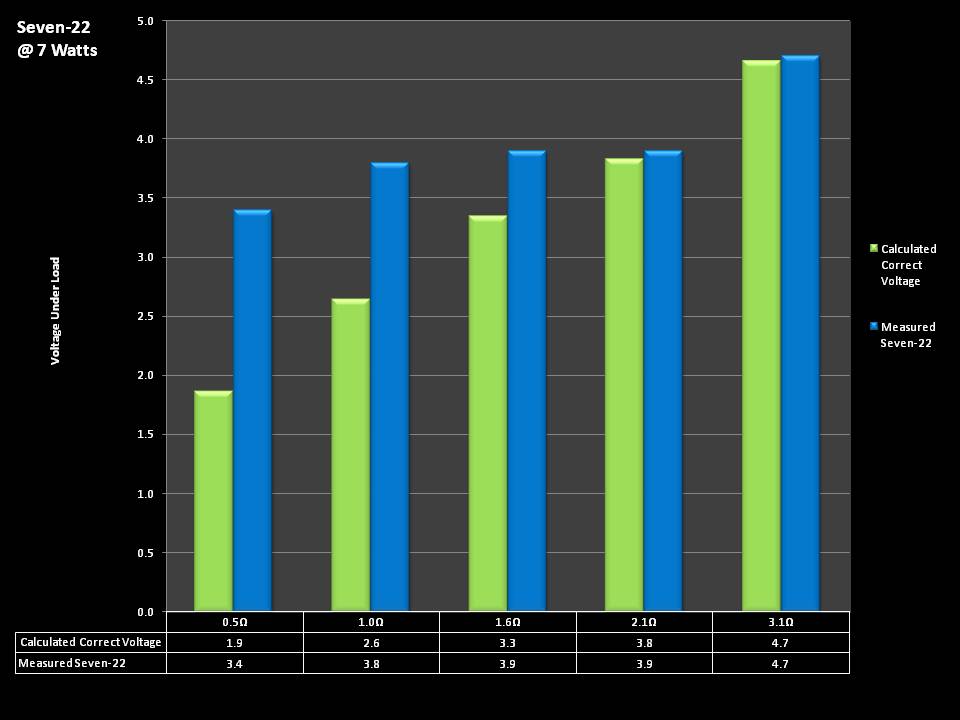



















 Store
Store












