Category: Recent News
A BATTERY MOOCH POST: Fake LG HE2 alert!
Fake LG HE2 Alert!
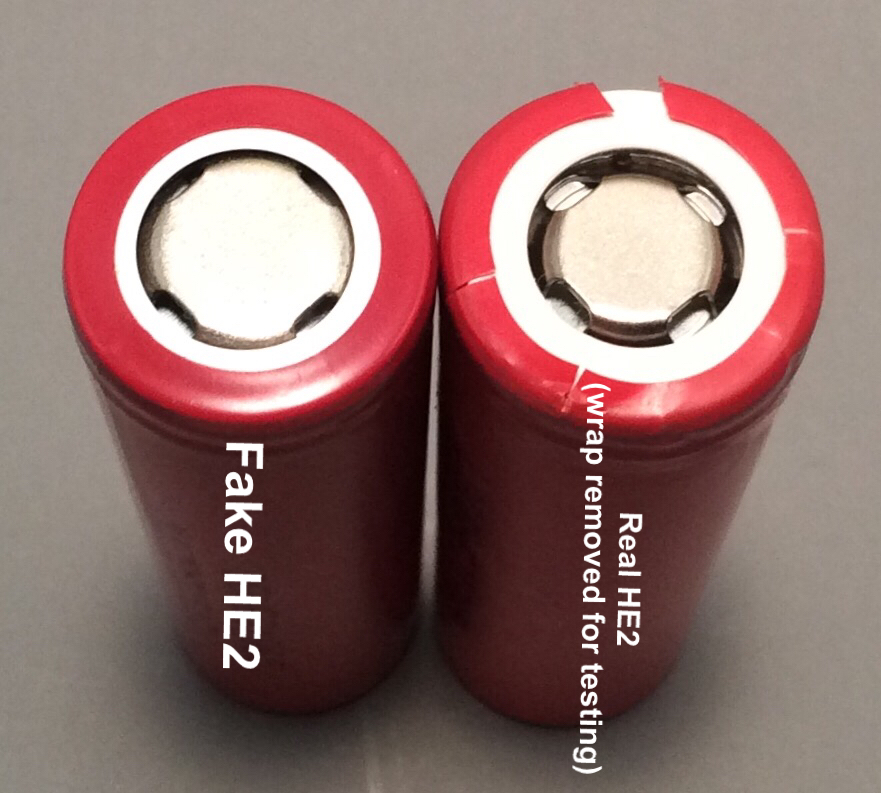
There are now fake LG HE2’s being delivered to shops. James Reeves of Vapors, Inc. was kind enough to send me two of the fakes to help put the word out. Thank you sir!
They also received fake HG2’s in the same shipment so still be on the lookout for those!
Here some info on the fakes…
- They omit “LG” from the start of the model number, the first line of characters on the wrap. The fakes just say “DBHE21865”.
- The top contact is larger than any LG top contact.
- The top cap seal, under the insulator ring, is blue and not the milky white it should be.
- The metal cans of the fakes don’t have the tooling marks near the top that the real ones do. The fakes are smooth-sided.
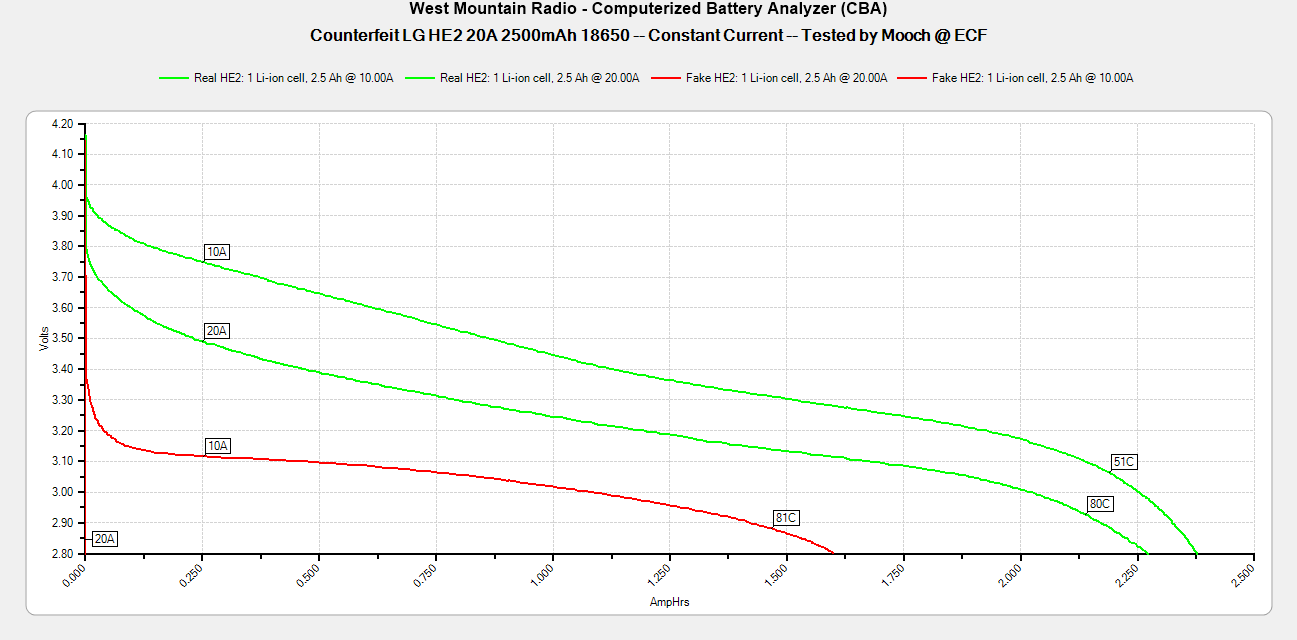
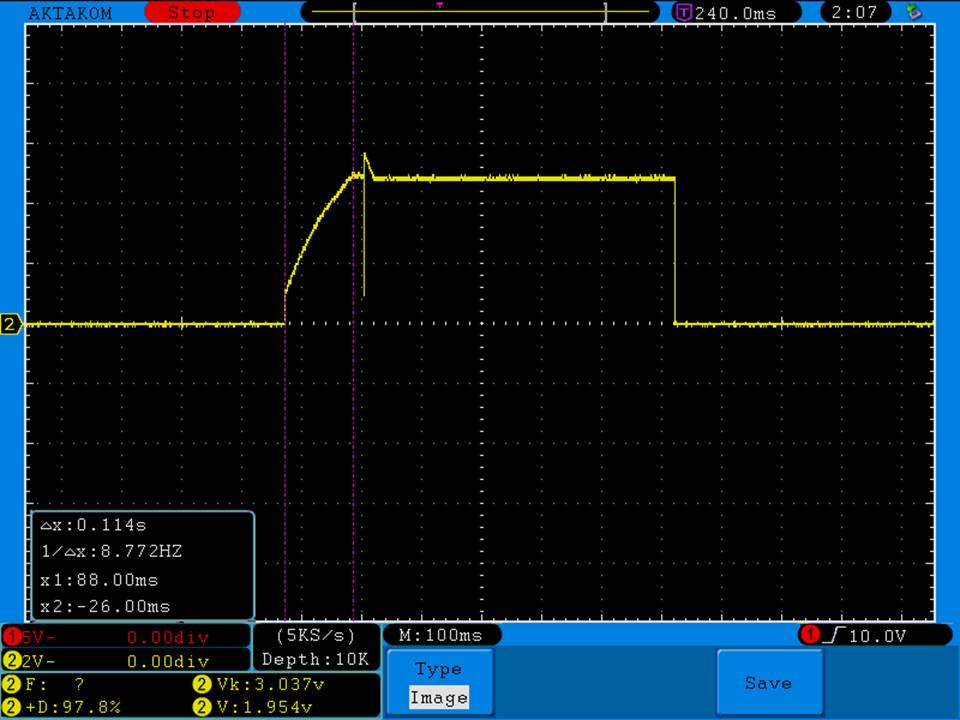
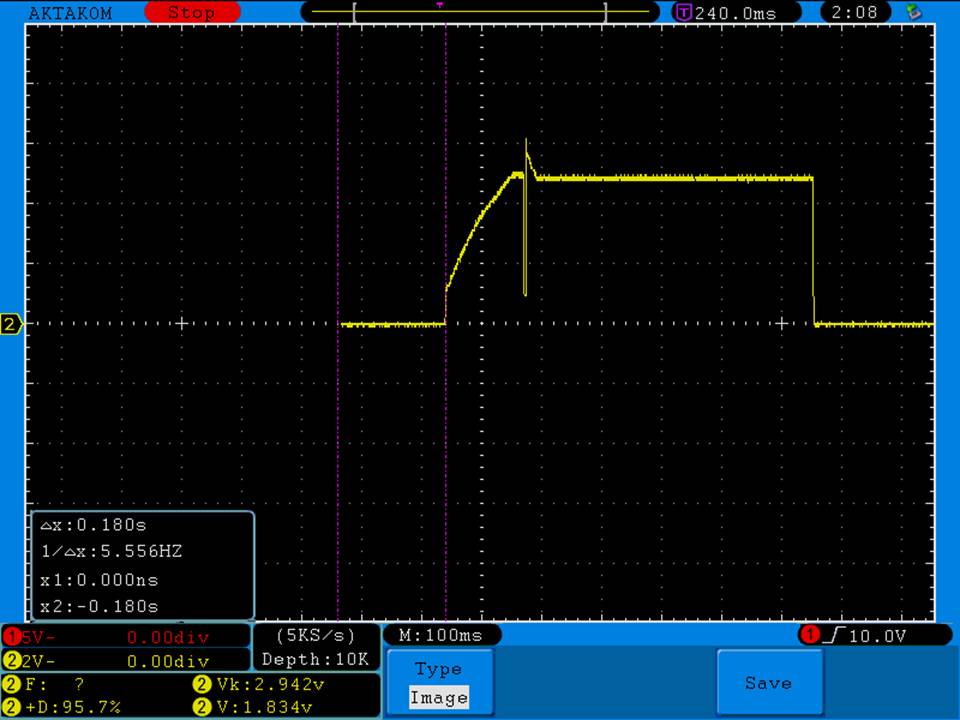
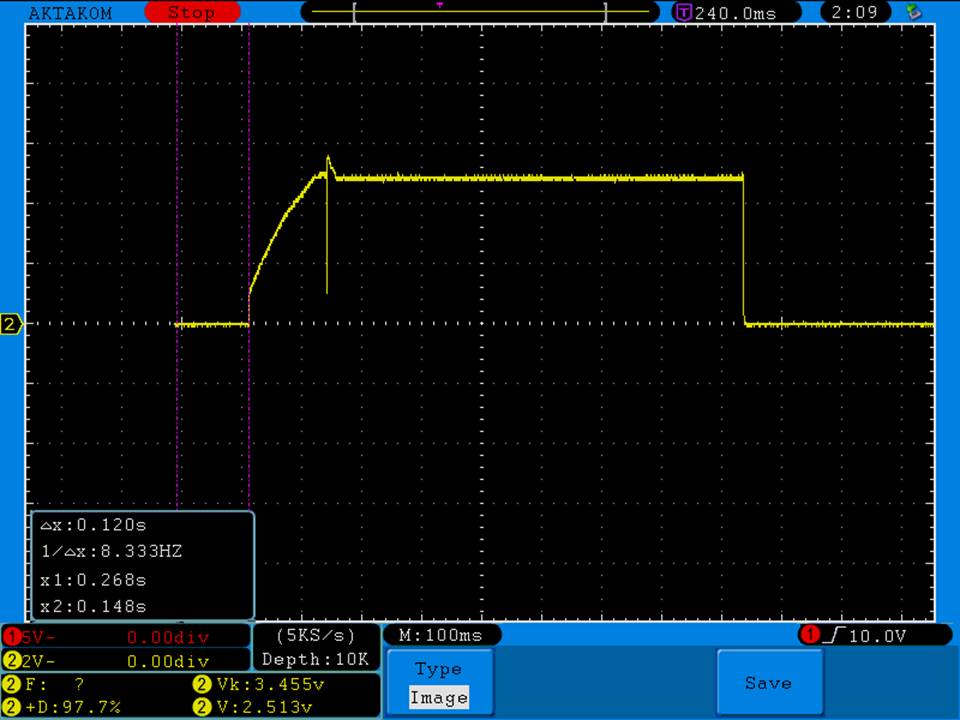
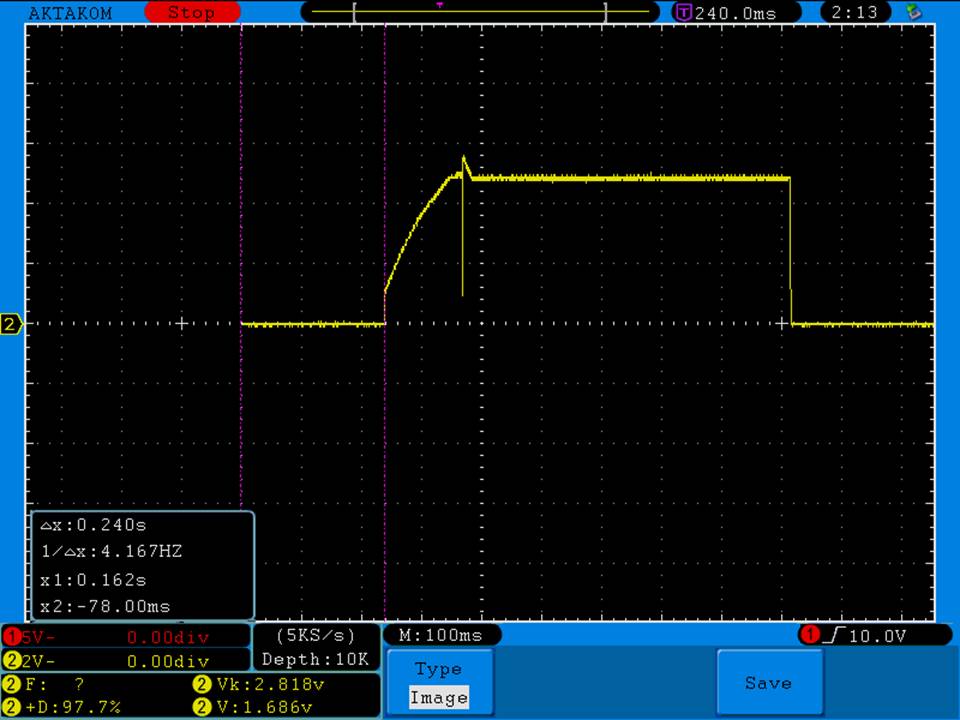
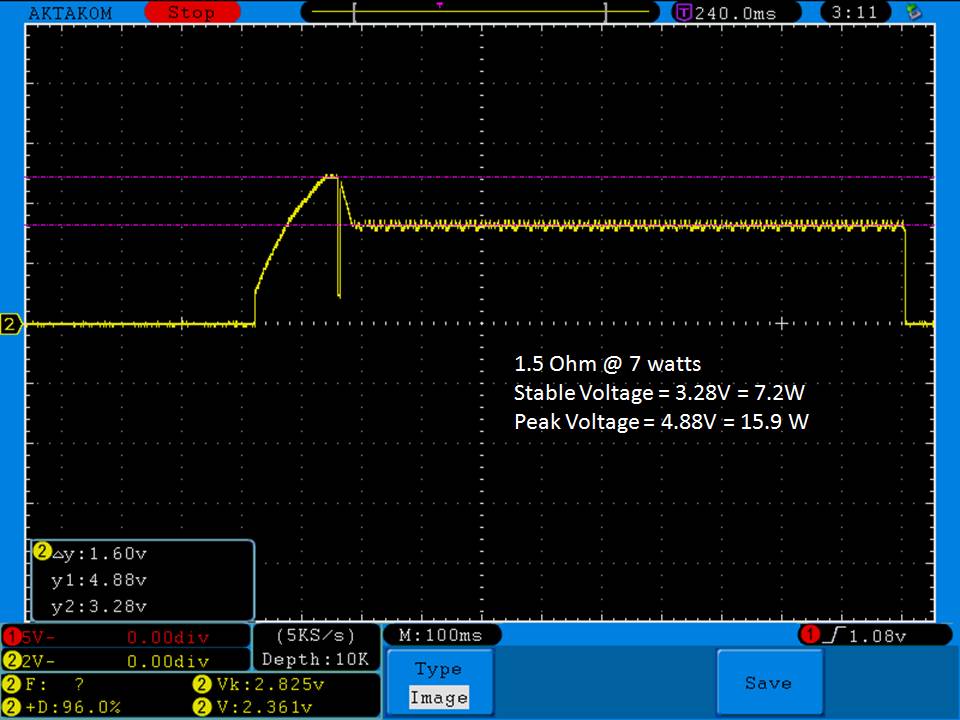
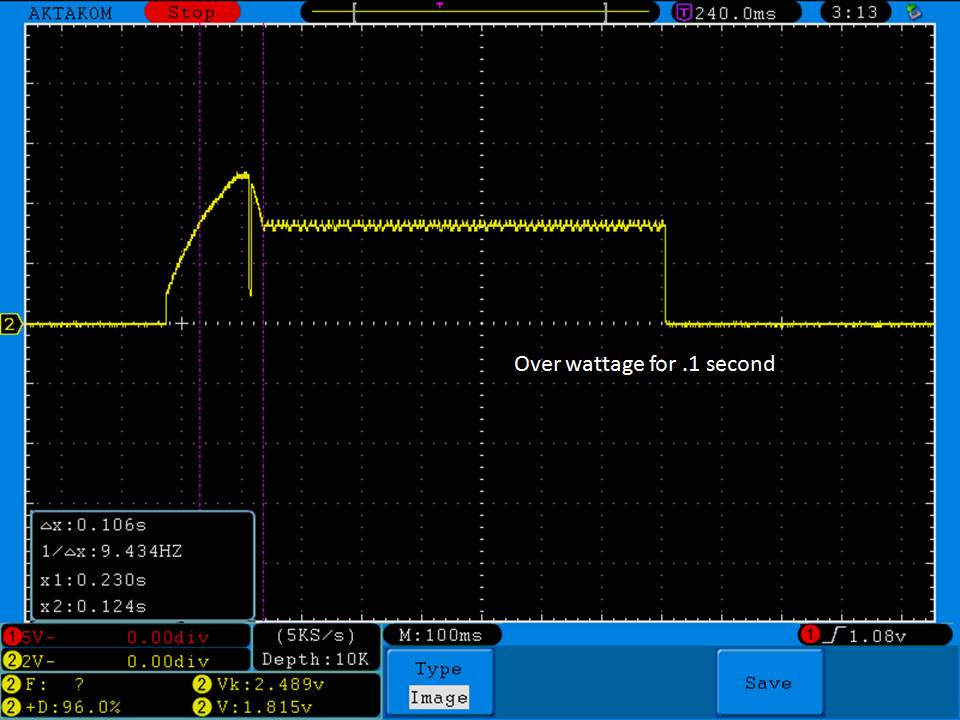
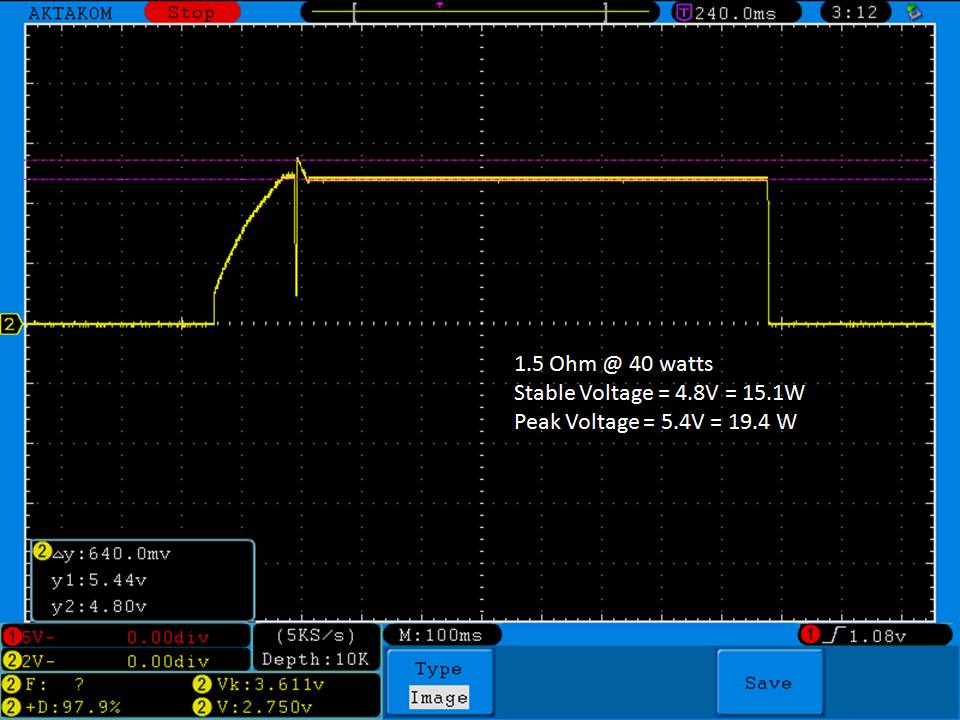
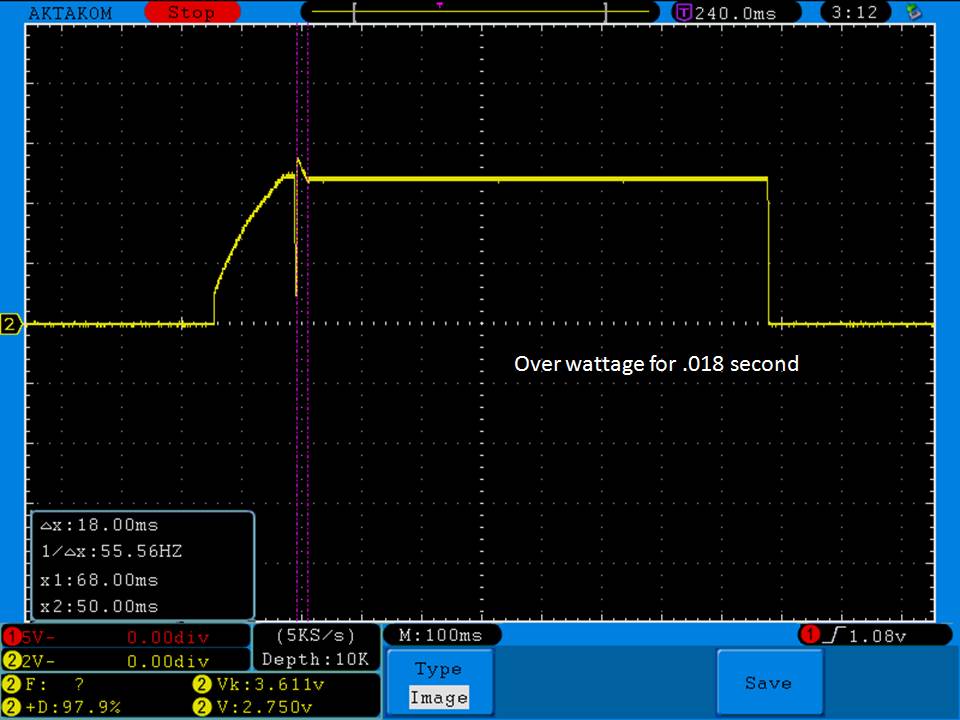
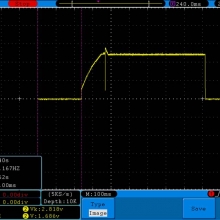
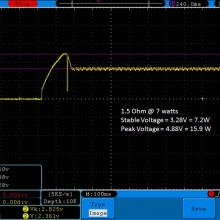
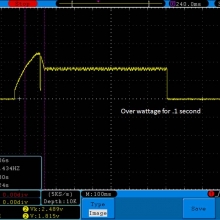
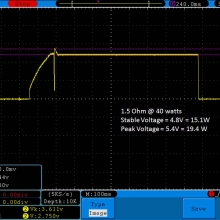
- Its performance is terrible. Even a 10A discharge sends the voltage plummeting.
Its internal resistance is over 70mOhms versus the approximately 25mOhms internal resistance of a genuine HE2.


From Kurt Loeblich, Owner of Cloud Chasers Inc. on A Billion Lives
Phil Busardo is correct. The Vapor Industry needs A Billion Lives. We need it now. Not in three weeks. Not in three months. Not in three years. Now.
In a perfect world, this film would already be available on iTunes, Netflix, and Amazon Video. You would be able to walk into your local Wal-Mart or Target and find it in their DVD section. Unfortunately, as everyone is well aware, we do not live in a perfect world.
We have become accustomed to vaping products being available relatively quickly after teaser pictures are revealed on social media. We’ve all done the group buys and pre-orders. We all have anxiously awaited shipment information for that new box mod. However, even then, the longest delays we have faced are those from the manufacturing process. Typically speaking, the longest we have to wait is a month or two.
The film industry is incredibly different than the vaping industry. Their industry has deep-rooted practices and traditions that protect the work and livelihoods of the producers and distributors. Typically speaking, there is a lengthy process that film-makers have to partake in order to have wide-spread distribution of their work.
Normally, a film will make its initial debut at a film festival. If they are lucky (or well connected!) they may be picked up by film distributor after their first event. However, this is not the usual case. A producer may need to take his film to numerous film festivals before they are noticed. U.S. premieres may not be enough. They may need to travel to many international film festivals before they can strike a deal with a distributor. Assuming a distributor selects their film, it is typically another year before the film makes its way to theaters.
Aaron and his team have done an incredible job at expediting the release of their film. They were able to complete their film in one year from start to finish. That is very much unlike typical films. Aaron and his staff put in thousands of hours and traveled extensively to make sure that by the time this film was completed, it would not be too late. If they would have followed standard business practices, this film would not have premiered until 2017, and it would not have been available until at least 2018.
Why can’t they just release the film on Netflix?
Why can’t it be available for download?
Why can’t we just have it for FREE?!?!?
As an industry, we need this film to have a huge impact. We need every news media outlet discussing it. We need to have elected officials watching it.
If this film was released for free (or via an online streaming service) the only people who would realistically be watching it would be us, the vapers. Sure, we’d try to get our elected officials to watch it, but without there being any “hype” behind it, there would be no true motivation for them to take the time out of their day.
The importance of various premieres across the United States is actually quite easy to understand. Aaron and his team plan on having Red Carpet events similar to that held in New Zealand on May 11th. With proper advertisement, the media will already be talking about it and elected officials may decide to view it for themselves. Not only that, but NON-VAPERS will get an opportunity to be introduced to the film, which is what we truly need. We have been fighting on the legislative and legal fronts for so long that we have all but forgotten about the general public. We need to remember them. Once we have their support, we stand a much better chance of changing the odds in our favor. An article by Mit Brickman discusses this topic quite well, which can be read at https://mitbrickman.com/2016/05/17/saving-a-billion-lives.
Why isn’t this in my local theater yet?
As previously discussed, there is a lengthy process to bringing a film to theaters. Unless the film maker has fifteen million dollars laying around, the only viable option with the lack of a distributor is launching the film at key locations for one time showings (the Red Carpet Premieres I brought up earlier). Unfortunately, those premieres are extremely expensive if they are to be done right. There needs to be advertising done via billboards, radio, newspaper, television… the list goes on and on. It doesn’t take long for these costs to exceed thousands upon thousands of dollars. Attention Era Media funded the production of this film by themselves. They could have asked for money from the vaping industry early on, but, in order to remain unbiased, they elected not to. However, now that the film is complete, and ready for premieres, they do need our help.
How can we help?
If Aaron wanted to take the conventional approach, it would be at least two years before this film makes it to theaters and DVDs. He could continue to show this film at film festivals until a distributor purchased the rights to the film. We all know, however, that we need this film to be available sooner rather than later. We need this film to be watched by politicians, doctors, and, more importantly, non-vapers. The only way to make this happen is to fund the premieres necessary to attract potential distributors. Unfortunately, at this time, a GoFundMe or Kickstart may not a viable option at this point. A crowdfunded film may have a negative impact on the likelihood of distribution. That being said, this is where our industry has to step in. If we want the film to make an impact now, before it is too late, we need to do whatever it takes to get this film into theaters. Incredibly, some companies have already stepped up to the plate and have pledged to fund some of these premieres. We need more companies to follow suit. For anyone interested in assisting in this matter, please send an e-mail to Jesse Hieb at Jesse@AttentionEra.com. With your help, we can make this happen.
Some Final Notes
For those of you who may be questioning my validity, I was fortunate enough to attend the New Zealand World Premiere, and I know the impact that it can and will have if we embrace it. I have been following the progress of this film since early on, and have been a supporter of not just this film, but of Aaron and his staff as well. I was granted the opportunity to spend a week with them in New Zealand, and I feel confident when I say that they are some of the most genuine people I have ever met. They have dedicated countless hours to bring this film to reality. It would be an injustice to not just them, but to A Billion Lives to not show them our support.
Kurt Loeblich
Owner
Cloud Chasers Inc.
Kurt@CloudChasersInc.com
Thanks very much for this Kurt. Hopefully vapers, shops, companies, and manufacturers will do what they can to help out. I’ll donate a bit to the cause as well. With our support, perhaps we can have this shown sooner rather than later. The time is now. It’s kinda important at this point.
Click the image for additional information regarding the movie.
Lost Art Liquids, LLC Files Lawsuit Against FDA
Here’s the second one (that I know of). Click it to get more information:
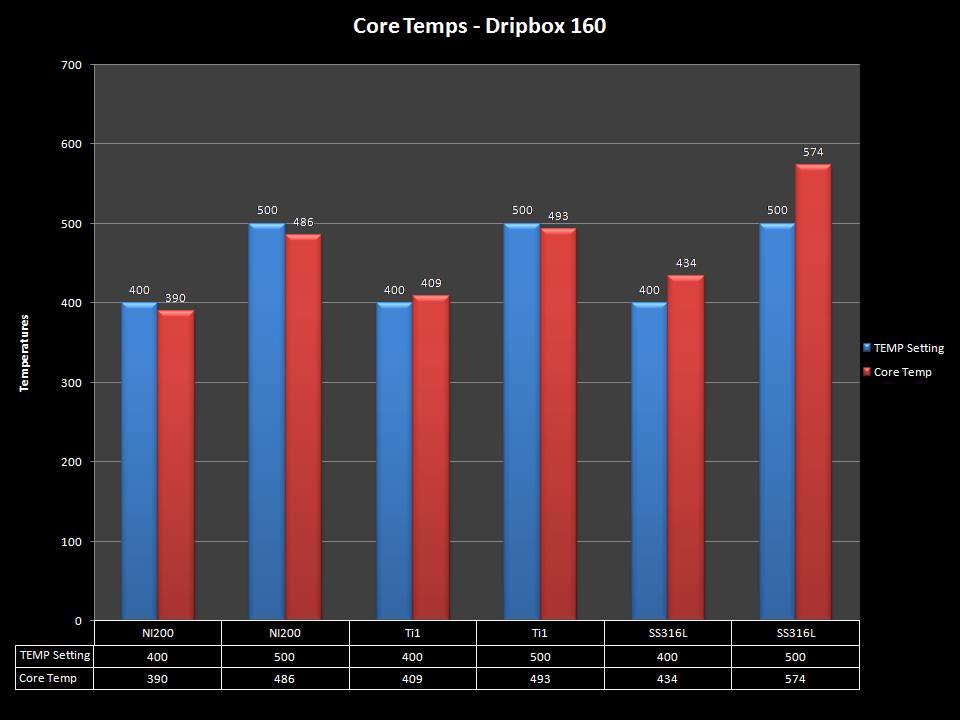
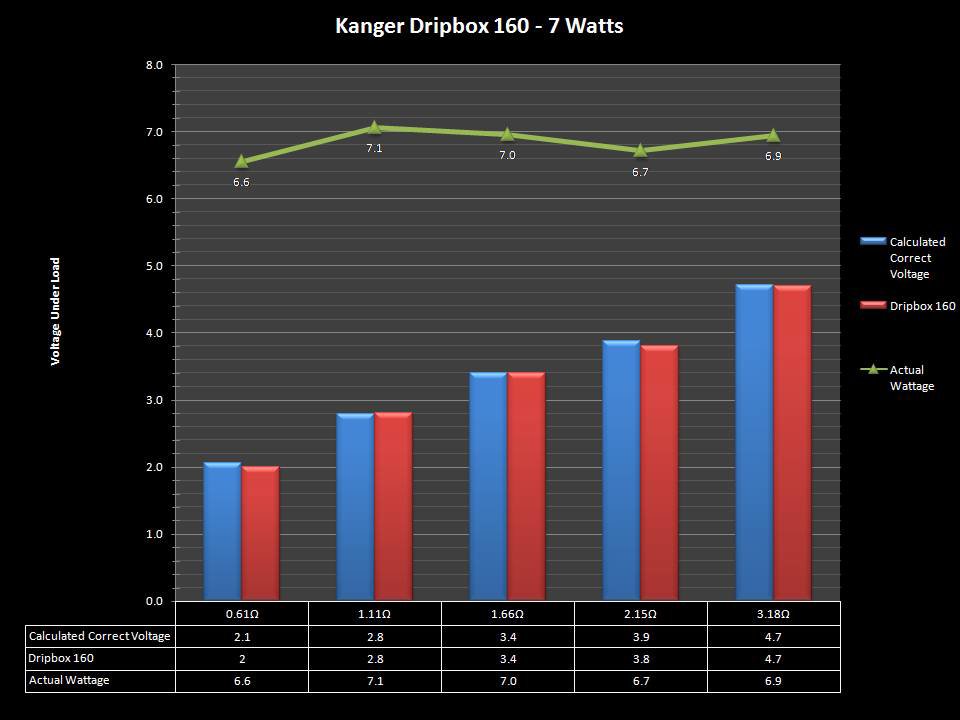
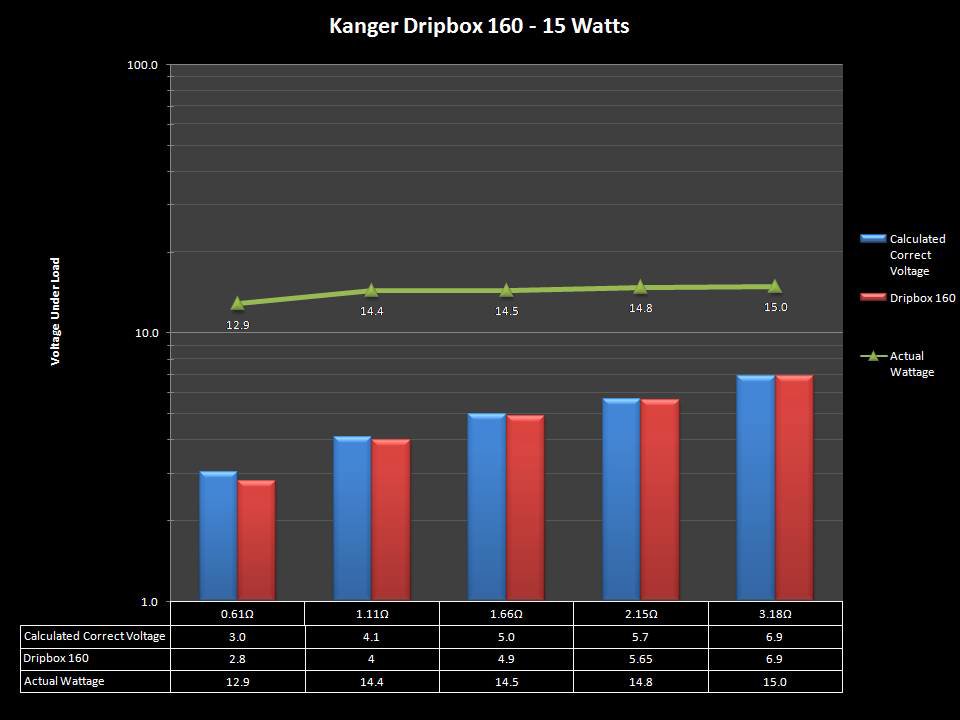
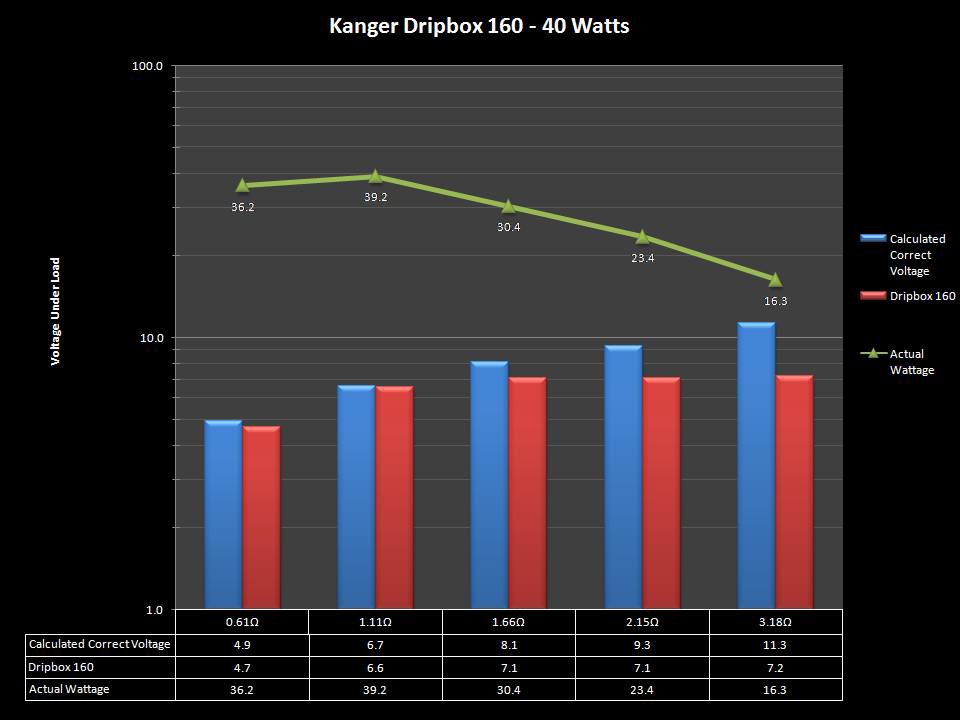
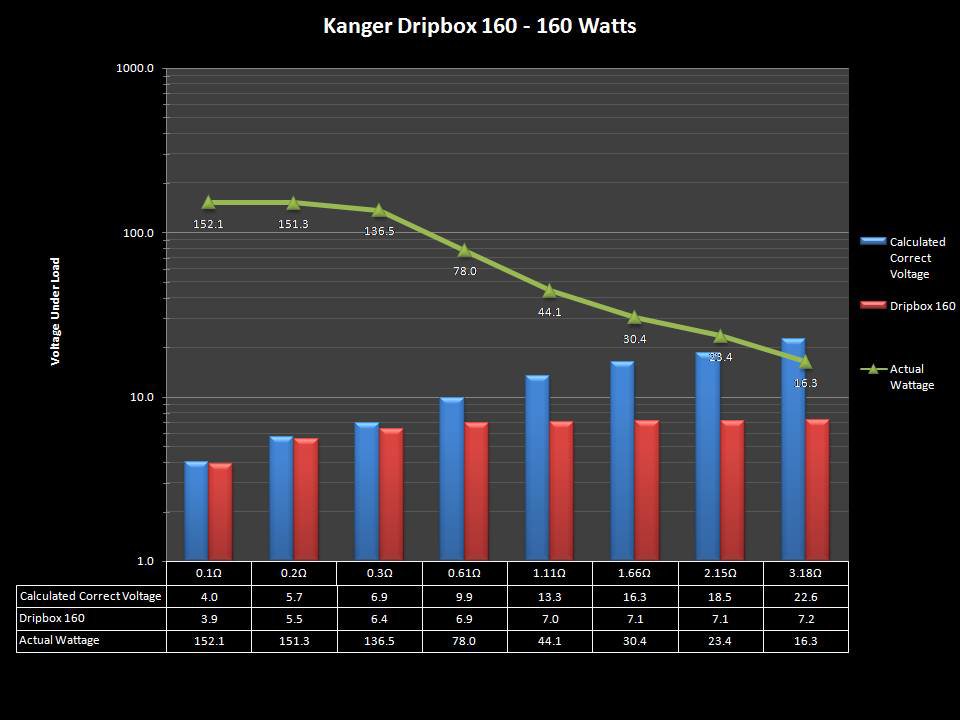
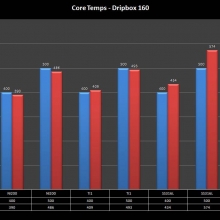
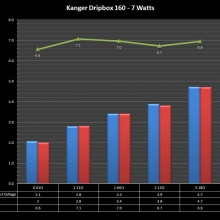
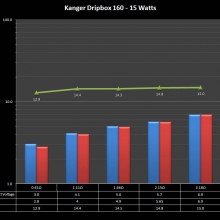
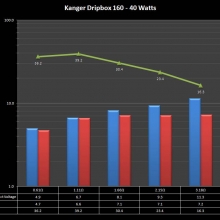
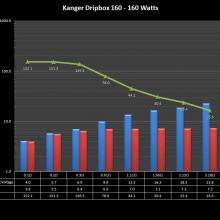
NEW IN THE QUEUE – THE KANGER DRIPBOX 160 – 5/19/16
The Kanger Dripbox 160 has arrived. Very impressed with the battery door on this one. It’s similar to the Cuboid door, but has a better spring loaded feel. The magnetic bottle door is also well done and uses stronger magnets than before.
For the shape on this one, as I mentioned in the announcement post, think the Reuleaux with the center battery replaced by a liquid bottle for squonking.
This one is regulated and does temperature control, features not found in the original Dripbox.
It looks like they’ve added a small air hole on the Subdrip 160 and have labeled it MTL. Not sure how well that’s going to work but we’ll see.
Here’s some additional information from Kanger:
Features:
- Easy DIY RBA base
- MTL and DL drip tip
- Juice capacity 7.0mL
- Disposal Dripping coil
- Replaceable dual 18650
- Squeeze to adjust your E-liquid
- Easy Juice filling, no more dropper
- 160W output with TC
In the box:
- Subdrip 160
- Dripbox 160
- DIY Drip base & clapton coil
- Replacement Drip coil(Kanthal)
- Extra juice tank
- Accessory pack
- USB cable
- Manual
From CASAA – PLEASE TAKE ACTION AND SHARE!
CASAA’s May 2016 Newsletter is coming soon, but, we wanted to make sure our members had some important information and engagement opportunities at their fingertips while we work out the finishing touches.
Here’s what you need to know for Thursday, May 19th, 2016:
A real petition that needs your participation:
Del. Larry Faircloth (R-WV) has proven himself as a valuable and effective advocate for vapor products in West Virginia. Now, in the wake of the FDA deeming regulations, he is taking his talent and legislative experience to lawmakers in D.C.
Please take action NOW by signing Del. Faircloth’s petition — which will be hand delivered to specific lawmakers — and support his effort to stand up to the FDA’s overreaching and disastrous regulations on vapor products.
Take Action – Sign the Petition
Survey – Centre for Substance Use Research
Last month, we alerted members to a survey produced by Dr. Christopher Russell, Senior Research Fellow at the Centre for Substance Use Research. The information gathered will help present to regulators, academics, and medical professionals a more accurate picture about how people are using vapor products and contribute to the conversation about how vaping can benefit public health. Dr. Russell is still collecting data and hopes to make this the largest survey of its kind — the more data the better!
Take Action – Complete the Survey
Change the predicate date for vapor products!
This week, CASAA updated our engagement in support of a change to the predicate date for products newly deemed to be tobacco by the FDA. We have made a slight adjustment to the letter we are asking members to send lawmakers to include support for both HR 2058 and the Cole-Bishop amendment. Please see our updated post here for more details. If you have not already taken action on this issue, please take a moment today to send a message to your lawmakers urging them to update the predicate date for vapor products.
Take Action – Send an Email
Update on the Deeming Regulations
Since the FDA deeming regulations were published for public inspection on May 5th, we’ve been reviewing multiple legislative and legal challenges to the rules. CASAA has joined with other groups in a coalition to explore all of these options as well as PR strategies. We are committed to combining our efforts to fight this battle on multiple fronts. This is an intensive process and, given the high stakes, we are thoroughly considering all viable pathways to challenging the FDA’s deeming regulations.
We are all aware of the desire for quick and decisive action in this matter. However, one hasty decision or poorly worded statement can create problems down the line. It is vital that any action is fully considered before being executed. A well-crafted and focused effort has the best chance for success. It is time to act with purpose, not react emotionally.
Some Good News
On Wednesday, May 18th, Sen. Ron Johnson (R-WI), chairman of the Senate Homeland Security and Governmental Affairs Committee, sent a letter to Food and Drug Administration (FDA) Commissioner Robert Califf raising concerns about the agency’s recent e-cigarette regulation, which could create undue burdens on small businesses and lead to negative unintended health consequences. We will continue to update you as this effort progresses. You can view the letter here.
If you are on Twitter, please also take a moment to retweet Sen. Johnson’s message to the FDA. Feel free to ask the @US_FDA and @FDAtobacco to respond in your own words — politely — and use the hashtag #AnswerRonJohnson
An Important Message From Gregory Conley, President of the American Vaping Association (AVA)
Over the next 27 months, there are going to be a lot of well-meaning (but ultimately dangerous) people that are going to try to promote events asking vapers to protest at the FDA, White House, Congress, etc.
Unless it is an effort that major advocacy groups are involved with, I strongly encourage you to not to participate in these events (and urge others to focus their efforts in more productive places). There are numerous potential problems with such an event, especially one that is hastily thrown together with no real plan.
Additionally,I want to even more strongly urge vapers not to fall for PR traps, like the FDA saying they’ll allow you to “interview” them. It will not turn out good. At all.
When such an event is proposed, please look into the person hosting the event. Is he or she really a positive representative of the vaping community?
Sincerely,
Gregory Conley
President — American Vaping Association
SMOKE FREE RADIO REPLAY – 5/17/16 – “Legislation, Litigation”
As promised, here’s the replay.
Strongly suggest you listen and share. Especially if you’re confused over the HR2058 Bill and Cole-Bishop Amendment.
*Ashley Davis, partner at West Front Strategies analyzes the HR2058 Bill & Cole/Bishop amendment, what they mean and what the industry and community need to do.
*Also Scott Eley joins us to discuss the industry led litigation against the overreaching onerous FDA deeming regulations.
JOYETECH JOINS SEVIA USA!
Very happy and proud to announce that Joyetech has joined the fight to save vaping as we know it and has become a member of SEVIA USA!
Following up on our most recent post, we have identified a few associations that we believe will have a positive influence, especially in helping us combat the FDA’s regulations.
Joyetech is proud to announce membership in SEVIA USA (a part of VTA, Vapor Technology Association) In addition to partnering with the Law Firm Pillsbury Winthrop Shaw and Pittman, Joyetech will be joining more organizations to support our cause.
Joyetech Group 18 May 2016
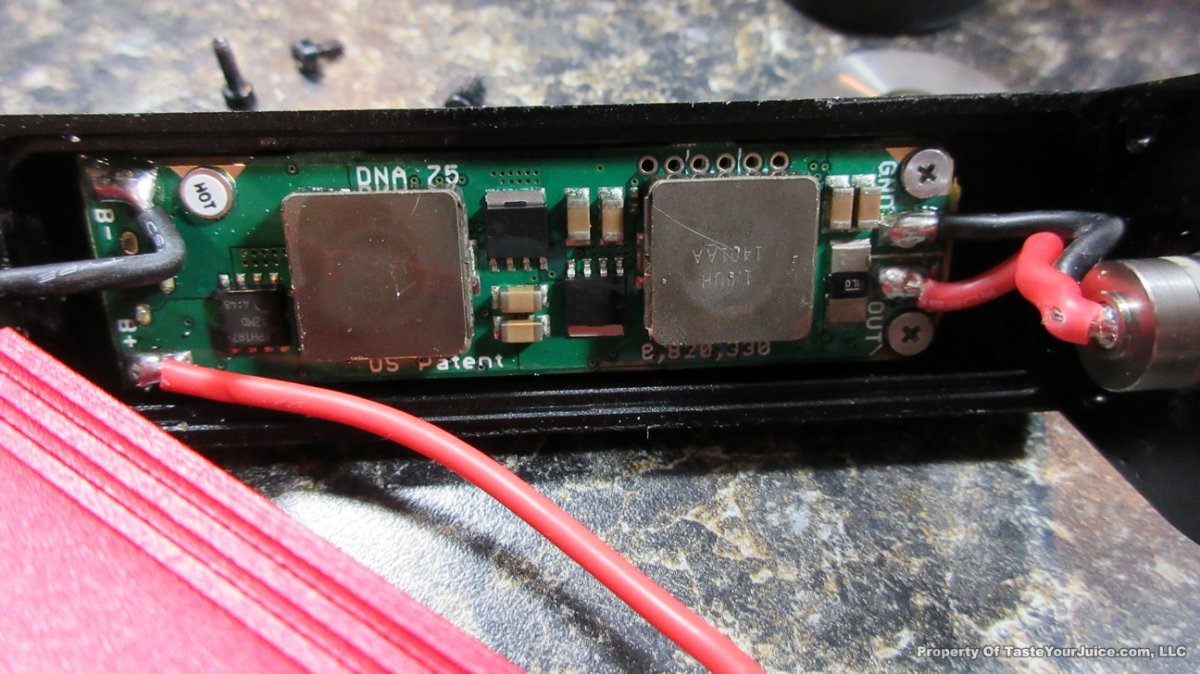
NEW IN THE QUEUE – THE HOTCIG DX75 – 5/17/16
Here come the DNA-75s! This is the second one that has arrived. Looks good and it’s a bit smaller than the Hcigr VT75 (see photos below).
Here’s a little more from Hotcig:
Colors:
- Red, black & grey
Main profiles:
- SIZE:87*42*30mm
- Thread Type: 510 thread
- Battery:18650/26650
- Output Mode: Ni200, Ti, SS316, VW
- Output Wattage: 75W
- Temperature Range: 100-300℃, 200-600℉
- Flashing LED fire button.
- Can be set up on EScribe software



































































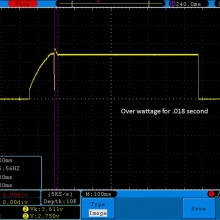
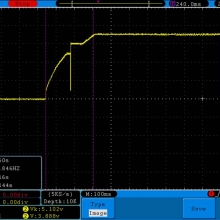
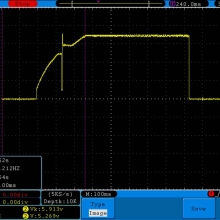
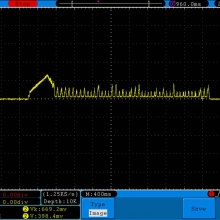













































































 Store
Store












