Author: pbusardo
FROM THE VTA – Critical PMTA Update

PMTA DEADLINE ARRIVES:
VAPOR INDUSTRY RESPONDS
September 9, 2020
This is a BIG DAY for the vapor technology industry. The day that so many said could not come has arrived. And, just like with every other deadline imposed by the FDA, the vapor industry has stepped up to the task.
Once thought impossible, already reports are coming in of many manufacturers filing their Pre-Market Tobacco Applications (PMTAs) covering what is sure to be thousands of products. Congratulations to all those companies who dug in, did the hard work, gathered the science and submitted their PMTAs by the deadline. Yes, companies had to make some tough decisions along the way, but that was always expected as we headed into the final phase of the Deeming Regulation.
Last week, the FDA confirmed it will take each applicant’s individual circumstances into account during the review process. To be sure, FDA already has been quickly replying to early applicants and issuing initial acceptance letters accepting applications for a variety of products.
While this may feel like a finish line, it is really only the beginning of an ongoing process with FDA as CTP reviews each application submitted and engages with further questions of the applicants.
VTA will continue our existing dialogue with FDA to strongly advocate for our members participating in the process. To that end, the process rolls out as follows:
Phase 1: Administrative Review. FDA initially reviews the application to make sure that basic requirements are in the application, it is in English, it includes required contact information and that it contains product identifying information. If these basic elements are included, a company should receive a letter from FDA accepting their application for further action. If not, FDA will send a “refuse to accept” letter.
Phase 2: Filing. FDA engages in a preliminary scientific review to make sure that everything required by the statute is included, such as health risk data, HPHC studies, descriptions of components, ingredients, additives, and principles of operation, description of facility controls, manufacturing, etc. If FDA finds these elements, a company will receive a letter accepting the application for filing. If not, FDA will send a “refuse to file” letter.
Phase 3: Substantive Review: This is FDA’s full-blown evaluation of all the science and data presented in each application. FDA will take recommendations from TPSAC and will engage with the applicant with questions. This process could take up to 12 months and during this time FDA may send the applicant a deficiency letter, giving them 90 days to provide missing or deficient information. If FDA has made a scientific decision to issue a marketing order, FDA will send an environmental information request letter before it can issue a final marketing order. Otherwise, FDA will send a “no marketing order” letter.
Phase 4: Post Marketing Reporting. As part of its approval process, FDA may require that the sale and distribution of your product is restricted (i.e., to protect kids). Or FDA may require the applicant to establish and maintain certain records and make certain reports available to FDA. Post market reports will vary based on submission and may include a requirement that the applicant engages in serious or unexpected adverse experience reporting or any manufacturing deviations.
VTA’s full, in-depth analysis summarizing the important developments for those impacted by the PMTA deadline, particularly as it relates to FDA’s enforcement now that the PMTA deadline has been reached, can be found here.
FROM REGULATOR WATCH – Deadline Day | Special Coverage of FDA’s Anti-Vaping Regulation
Here’s the latest from Brent Stafford at Regulator Watch:
The U.S. Food and Drug Administration’s anti-vaping regulation takes effect today Wednesday, September 9, and promises to deliver a lethal blow to the U.S. vaping industry.
Guy Bentley, Dir. of Consumer Freedom at the Reason Foundation joins RegWatch to discuss the impact of the industry killing Premarket Tobacco Application Process; how the FDA’s anti-vaping regulation forces winners and losers; and why “clear the market” is an extraordinary response.Live Streamed: September 9, 2020
Sr. Producer: Cindy Schmidt
Exec. Producer: Brent StaffordThis episode is supported by: DEMAND VAPE
Make RegWatch happen, go to: support.regulatorwatch.com
DP SHOW REPLAY – THE UVA SAVE THE VAPE WASHINGTON DC RALLY RECAP
The DP Show! Phil & Dimi go to Washington! The “Save The Vape” Rally Recap
S01E12
In this video we recap the UVA Save The Vape Rally in Washington DC.
You’ll get to hear the speeches made by Dimitris and Phil.
We’ll talk about the PMTA deadline.
Then we have some fun and go for a motorcycle ride.
As always we take your calls and questions.
VAPE WHEEL PRIZE DONATORS:
Big Willie’s
Dash Vapes
Eleaf
Five Pawns
Innokin
Joyetech
Lunar Rover
Nixteria
Vaporesso
THE VIDEO:
THE PODCAST:
COMING SOON!
BILL 810 VETOED BY GOVENOR RON DESANTIS! E-LIQUID FLAVORS FOR ADULTS CONTINUE IN FLORIDA!
“which is superfluous given this is already mandated by federal law”
Hmmm… who has been screaming that for a while now? 😉
- THANK YOU Ron DeSantis for doing the right thing and not being a Cuomo!
- THANK YOU Ron DeSantis for making sense!
- THANK YOU Ron DeSantis for understanding that adults also enjoy flavors and for understanding small businesses and lives depended on this decision!
- THANK YOU to Nick Orlando and the rest of the FSFA for your tireless efforts with this.
- THANK YOU to everyone else who was involved with this result.
Looks like I made the right decision moving out of NY for more reasons than one!
On a sadder note… There goes my black market business plan and we’re probably going to lose the deposit on the submarine.
PLEASE!!! Tweet Ron DeSantis (@GovRonDeSantis) and thank him for this!

TONIGHT AY 9PM EST ON THE DP SHOW!
From Tony
Phil,
I just wanted to take a moment of your time to say thank you for the Innokin Sceptre deluxe kit that you sent me last week, it is really a great AIO.
After over seven years vaping it amazes me on how far things have come and where it might be allowed to go if the anti’s don’t get their way.
I appreciate all the work that you and Dimitri do for our community and working to help people get away from cigarettes and finding a safer alternative. Thank you for fighting for all of us.
Sincerely,
Tony
FROM REGULATOR WATCH – One Voice | Vapers Rally to Save the Vape, Washington, DC | RegWatch
Here’s the latest from Brent Stafford at Regulator Watch:
For the second time in less than a year, vapers are set to descend on Washington, DC to call on President Trump to intervene and put a stop to the U.S. Food and Drug Administration’s industry killing Premarket Tobacco Application Process.
The rally is scheduled for September 5th, just 4-days before the PMTA deadline and organizers hope a strong showing by advocates and grassroots vapers will pressure politicians to “do the right thing” and support flavored vaping products as a tool for harm reduction.In this episode of RegWatch we are joined by Tristan Thompson and Sherwin Mena from the United Vapers Alliance. Hear the plans for the coming rally, learn how you can make a difference, and ask the organizers questions in advance of the big day.
Only on RegWatch by RegulatorWatch.com
Live Streamed: August 31, 2020
Sr. Producer: Cindy Schmidt
Exec. Producer: Brent StaffordThis episode is supported by: DEMAND VAPE
Make RegWatch happen, go to: support.regulatorwatch.com
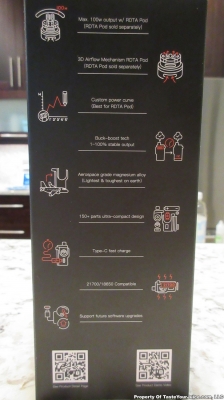
THE RINCOE MANTO MAX 228W KIT
A PBusardo Review – Vapes fine, but technically… The Rincoe Manto Max 228W Kit
It does what it does pretty good, just don’t look under the hood too much.
We take a look at the Manto Max 228W Kit from Rincoe and then I let you know how to win one!
The Links:
Rincoe
VapeSourcing
VapX
The Video:
*NOTE: Any use of these videos in part or in their entirety without Phil Busardo’s expressed written consent is strictly prohibited.
The Photos:
Thank you to and please support the Premiere Taste Your Juice Sponsor…
From Lindsay
The Smokers Show S02E21
I just wanted to thank you for bringing such an excellent point to the table. Your episode about Vaping News gives people a chance for people’s eyes to be opened and see behind the curtain a little bit. This is something so much bigger than vaping, and it is damaging the way people receive information and therefore changing their opinions on things. It is manipulative, and it very dangerous.
Using fear-mongering, and presenting vaping as dangerous we are cloaking agendas as news stories. We wait for a story or event to use and propagandize in this case to fuel the anti-vaping fires.
I just think that is awesome that you have allowed people to see this in a way that related to them with vaping. This is happening with news stories about everything, and maybe it will make people second guess the next story about politics, products, etc.
We don’t have trust anymore, we just have agendas, it seems just like more and more manipulation every day. I will continue to listen to your show and am so glad I found it. Keep it up!
Lindsay
THE VAPX GEYSER
A PBusardo Review – They said it does, but really, it doesn’t – The VapX Geyser
What was going to be a review, became more of a Show-N-Tell. Let’s check out the VapX Geyser.
We’ll also give one away and find out who won the last “Not A” Contest.
The Links:
United Vapers Alliance
Bull City Flavors
VapX
3AVape
Freemax
The Video:
*NOTE: Any use of these videos in part or in their entirety without Phil Busardo’s expressed written consent is strictly prohibited.
The Photos:
Thank you to and please support the Premiere Taste Your Juice Sponsor…











































































































































 Store
Store












