Author: pbusardo
SEVIA USA Contributes to the Industry Lawsuit
On behalf of the E-Vapor Industry, Keller and Heckman filed a Complaint in the U.S. District Court for the District of Columbia challenging portions of FDA’s Deeming Regulation and the Tobacco Control Act on various constitutional and administrative grounds. The named Plaintiffs in the lawsuit are:
1. Right To Be Smoke Free Coalition
2. American E-Liquid Manufacturing Standards Association
3. American Vaping Association, Hoboken, New Jersey
4. Electronic Vaping Coalition of America, New Berlin, Wisconsin
5. Georgia Smoke Free Association, Fort Oglethorpe, Georgia
6. Kentucky Vaping Retailers Association, Inc., d/b/a Kentucky Smoke Free Association, Louisville, Kentucky
7. Louisiana Vaping Association, Chalmette, Louisiana
8. Maryland Vape Professionals, LLC, Baltimore, Maryland
9. Ohio Vapor Trade Association, Miamisburg, Ohio
10. New Jersey Vapor Retailers Coalition, Roseland, New Jersey (http://www.njvaporcoalition.org/)
11. Tennessee Smoke Free Association
Also supporting the lawsuit are SEVIA USA and CASAA, AVA, SFATA and NBS.
In the Complaint, we argue the following:
(1) Count I – Violation of Administrative Procedure Act – Grandfather Date: FDA had the authority and the statutory duty to establish a new Grandfather Date for e-vapor products (ENDS) or apply its enforcement authority so that some ENDS manufacturers, including e-liquid companies, would have the opportunity to forego the Premarket Tobacco Application (PMTA) pathway and avail themselves of the option to submit Substantial Equivalence (SE) Reports. By not doing so, FDA violated the Administrative Procedures Act.
(2) Count II – Violation of Administrative Procedure Act – Pre-market Authorization Process: FDA was obligated to consider the continuum of risk of tobacco products and exercise flexible enforcement authority mandated by Congress, instead of a “one-size-fits-all” regulatory regime treating ENDS the same as cigarettes and other harmful products and forcing ENDS manufacturers into the PMTA process, which will all but ban the entire e-liquid and device categories. Accordingly, FDA’s application of the PMTA process to ENDS products violates the Administrative Procedures Act.
(3) Count III – Violation of Due Process and Equal Protection Clauses – Tobacco Control Act: In the Tobacco Control Act, Congress made clear that different tobacco products present different risks and that FDA should exercise its enforcement authority in a flexible manner. But if, as FDA argues, the Agency is mandated to enforce a “one-size-fits-all” regime to all products, including less harmful ENDS, than Congress did not provide FDA with the necessary tools and regulatory flexibility to achieve the Tobacco Control Act’s stated goals, which include allowing newer and safer products to enter the market. As a result, the Tobacco Control Act is unconstitutional under the Due Process and Equal Protection Clauses.
(4) Count IV – Violation of First Amendment and Administrative Procedure Act – Ban on Free Samples: FDA does not have a substantial interest in prohibiting access to free samples of ENDS products by – including taste testing e-liquids in vape shops. The complete ban on free samples does not directly advance the government’s interests. There were more narrow options available to FDA to advance their stated interest in preventing youth access while still allowing vape shops and others to market using free samples. Accordingly, the total ban on free samples violates the First Amendment and the Administrative Procedures Act.
(5) Count V – Violation of First Amendment and Administrative Procedure Act – Modified Risk Tobacco Products: The Modified Risk Tobacco Product (MRTP) provision of the Tobacco Control Act, as applied to ENDS products – which do not produce smoke, combust e-liquid when used as intended, or produce aerosol that contains the harmful substances found in tobacco smoke – does not advance any purported government interests (which focus on traditional tobacco products), and captures commercial and non-commercial speech that is clearly not misleading (e.g., smoke free claims). By applying the MRTP provision and its extensive review process to ENDS products, FDA violated the Administrative Procedures Act and the prior restraint doctrine.
(6) Count VI – Violation of Administrative Procedure Act – Definition of “Tobacco Product” and Application to ENDS: FDA considers a broad range of ENDS products to be regulated as tobacco products or “components or parts,” including software that operates devices, batteries, displays, tanks, etc. FDA intends to regulate these products as tobacco products despite the fact that they do not contain tobacco, are not derived from tobacco and are not components and parts of an actual tobacco product. There is nothing in the legislative history of the Act, and FDA has provided no supporting rationale, for why these items should be regulated as tobacco products merely because they are used to consume the product. Accordingly, FDA’s application of the Tobacco Control Act’s definition of “tobacco product” to certain ENDS is unreasonable and unlawful under the Administrative Procedures Act.
(7) Count VII – Violation of Regulatory Flexibility Act – Unlawful Cost/Benefit Analysis: The Regulatory Flexibility Act requires administrative agencies to consider the effects of their regulatory actions on small business entities. FDA failed to consider significant alternatives, including, but not limited to, the impact of any compliance period on the ability of small entities to successfully navigate the PMTA process given that FDA concedes that there are no long-term clinical studies or other data necessary to support such applications. FDA only considered several, modest alternatives focused on discrete issues like labeling burdens. The Agency did not make a reasonable, good faith effort to consider alternatives that would have an overall impact on all small entities. In short, FDA substantially overestimates the benefits of the Deeming Rule and underestimates the costs. Accordingly, the Court should take corrective action, set aside and remand the Deeming Rule to FDA, and defer enforcement until the Agency complies with the Regulatory Flexibility Act.
(8) Count VIII – Violation of Administrative Procedure Act – Unlawful Cost/Benefit Analysis: The Tobacco Control Act makes clear that FDA was required to adequately consider the costs and benefits of the Deeming Rule. As with the Regulatory Flexibility Act, FDA failed to consider regulatory alternatives, such as an extended compliance period, that would have significantly increased the chance that ENDS manufacturers would be able to comply with the PMTA process, thus avoiding what will be close to an effective ban on ENDS products. The Agency also failed to properly estimate key factors necessary to an adequate cost/benefit analysis, including the number of entities and products affected, as well as the number of PMTA applications that will be filed. For many of these numbers, FDA did not adequately explain or support its conclusions. As a result, FDA violated the Administrative Procedures Act and, therefore, the Deeming Rule must be remanded to the Agency so that a proper cost/benefit analysis may be conducted.
Full complaint can be found here: http://www.khlaw.com/Files/27053_FDA_Deeming_Rule_Complaint.pdf
Dimitris and I would like to thank everyone involved, especially the SEVIA USA founding members which were instrumental in getting this lawsuit off the ground.
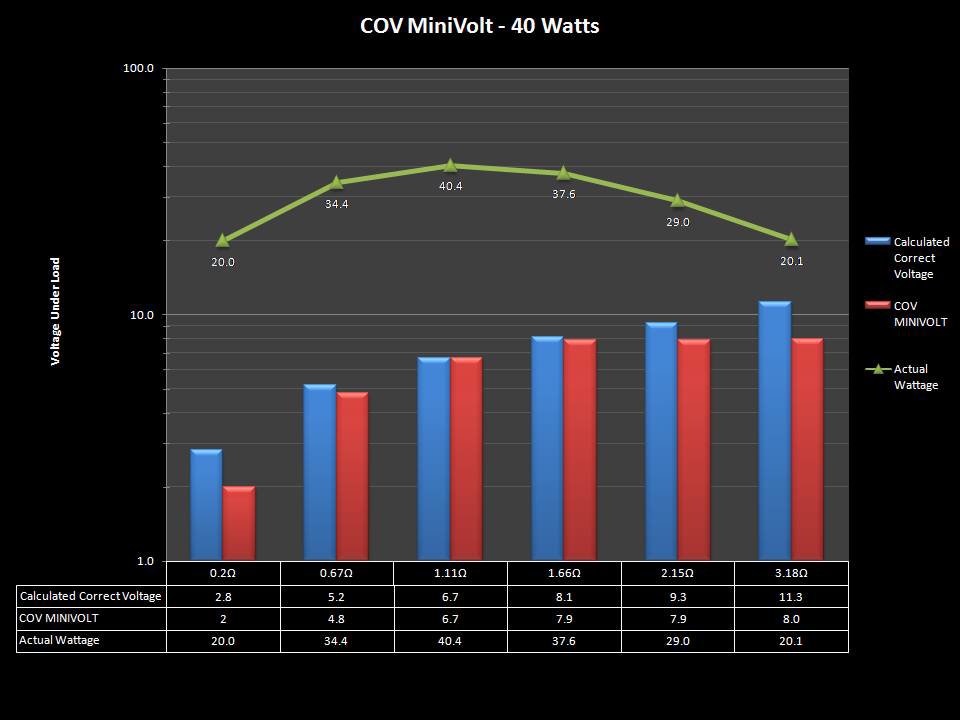
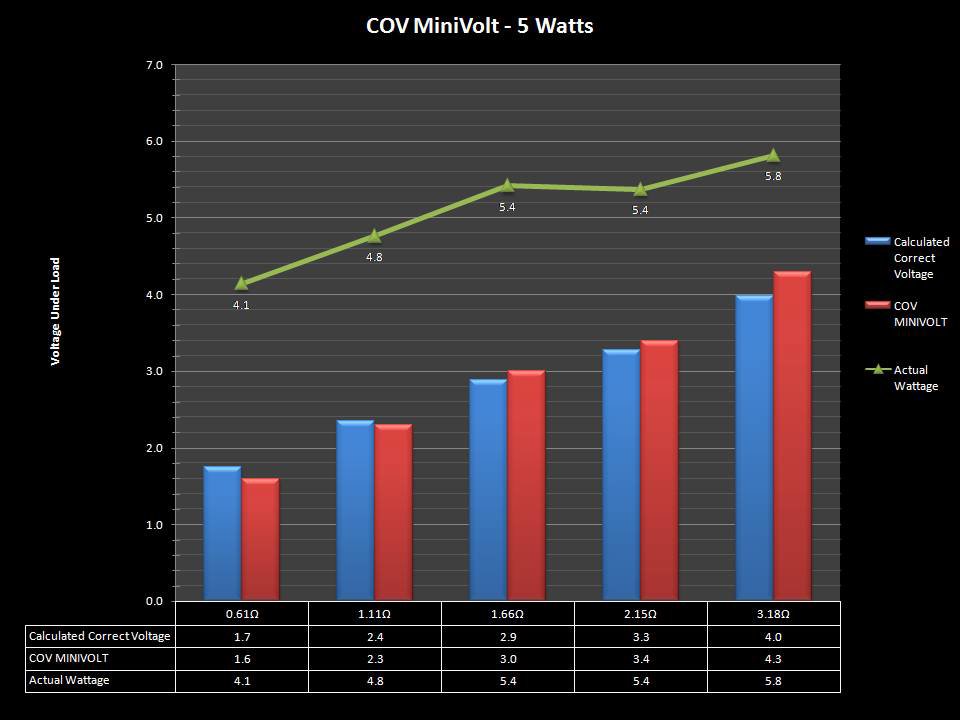
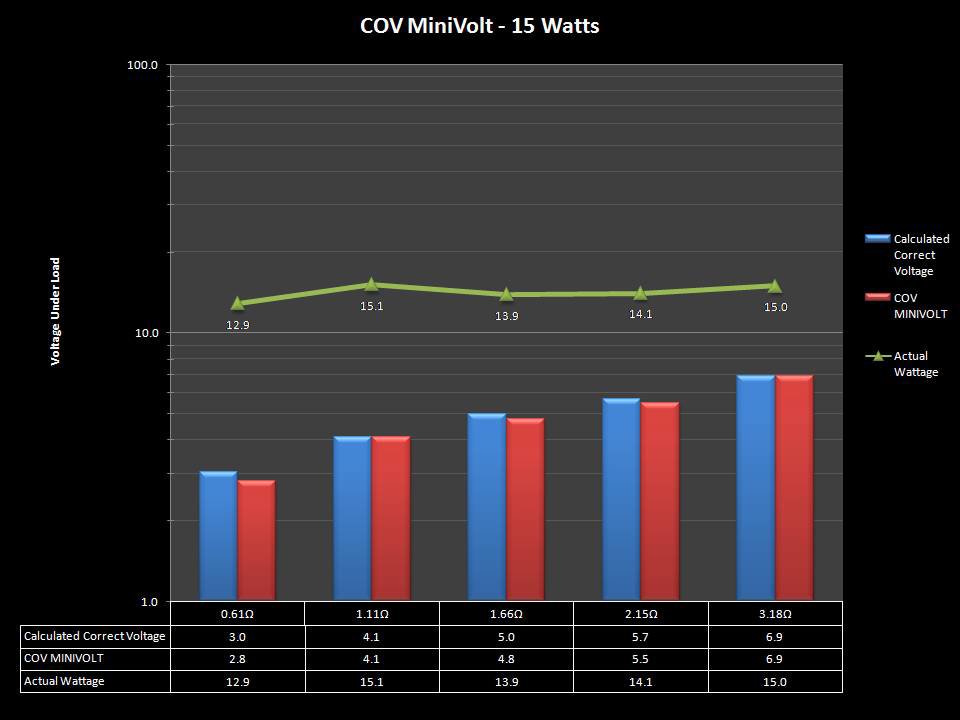
THE “MINI MARATHON” BEGINS… FIRST UP, THE COV MINIVOLT & LAST CONTEST WINNER(S)!!
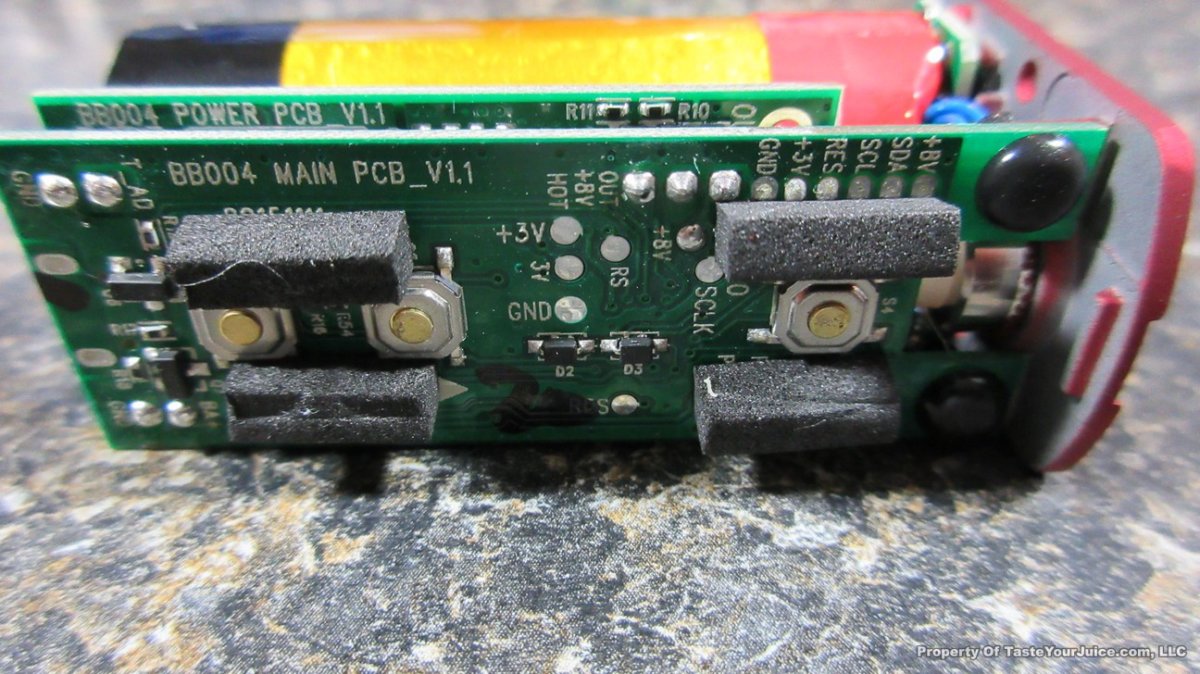
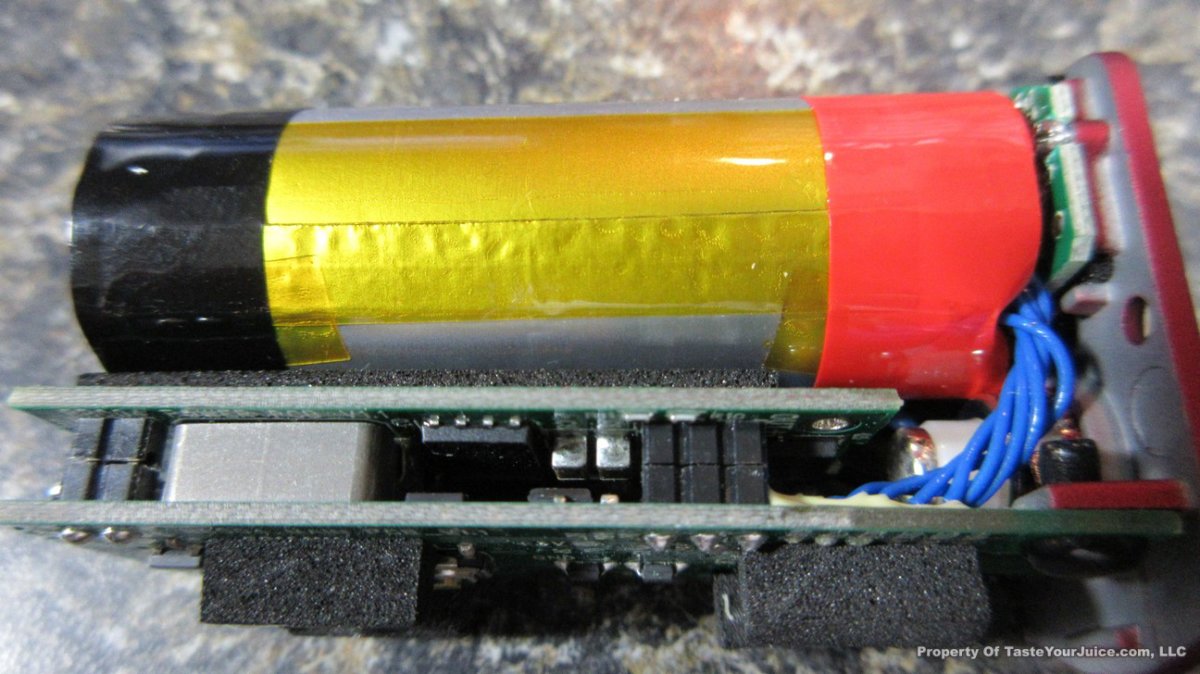
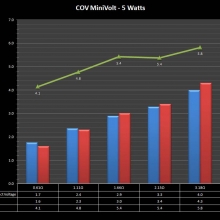
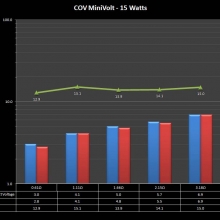
A PBusardo Review – The Mini Marathon Part 1 – The COV Minivolt
We kick off the “Mini Marathon” with this video. A look at some of the miniature devices coming out. Part 1 is a (not so complete) review of the COV Minivolt.
At the end we find out who won the last contest.
The Links:
The Council Of Vapor (COV)
Steeping Giant
Aspire
Kanger
The Video:
*NOTE: Any use of these videos in part or in their entirety without Phil Busardo’s expressed written consent is strictly prohibited.
The Photos:
From Eric
Hi Phil ( I called you Pete the last time I contacted you -sorry – I think
that it happens a lot to you for some reason). I just wanted to drop you a quick
line because I caught your appearance on the Vaping Dusk till Dawn from 3/16 ( I
know – I’m way behind on my viewing). Great show. It was great to hear more about
your journey and life and not just about vaping gear. I love your reviews and your
passion and information about vaping has changed my life. I haven’t had a cigarette
in over 4 years. Better than that, I love vaping. It has become more of a hobby
rather than an addiction. You have helped me more than you could know with all kinds
of info over the years. Anyway, somehow I feel like we’re friends that just haven’t
met yet. You talked on this show about how work, family, reviewing, etc leaves you
little “Phil time. Please remember my friend, taking care of yourself includes
granting yourself permission to take some time to goof off any old way that you’d
like to. I (and all your vaping friends) can do with a few less reviews as long as
we still have a healthy, happy and passionate Phil giving us the real info on vaping
gear. Harm reduction also has a very personal and emotional side. Take time to
“smell the roses. We’ll wait until you get to the next review. Rock on DJ Miami.your friend
Eric
From Carlo
Hi Phil how are you my name is Carlo you recently attended the CVE in Toronto and met my friend Danielle I admire your knowledge of the Vaping world I was floored when Danielle show me that video I want to express my gratitude for the knowledge that you have provided me and everyone in the Vaping community also would like to say that you are extremely influential and knowledgeable in what you and your colleagues do to provide us with information I thank you for that. PS
you and Dimitri look cute together hope to hear from you soon and please let’s continue on this relationship ensuring knowledge and empowerment to those who need it most oh and by the way the cruise was phenomenal I’m Italian and I love to eat take care hope to hear from you soon
From Richard
Hi phil just want to say thanks for everything you do for us vapors. I can see you lead a very busy life. And for you to go out of your way for these great vids and events im very grateful. Thanks for pushing so hard to inform all of us. Im an ex smoker vaping for 4 yrs. Now and I live not far from you Im in buffalo ny. Would love to get one of my mods signed by you some day. Thanks for inspiring so many people.
From Chris
Hey Phil,
My name is Chris Kunkel. I’m a 32 year old father of three and I have been a fan of your videos for a little over a year now. I’ve seen other success stories posted on your Taste Your Juice blog, and felt it was time to share my own. With all of the negative publicity out there and the deeming regs being published, I felt that if my story can help others like me, that it was time to tell it.
I had my first cigarette when I was 11, stealing from the pack of Virginia Slims my mother had. I smoked off and on through middle and high school to appear rebellious and cool, stupid I know. It was in 2002 when I started full time. I had just graduated high school and had enlisted in the army. I found myself at 18 stationed in Korea, half a world away from my family and practically anyone I knew. I was alone for the first time in my life and I picked back up smoking to deal with the stress. For over 13 years, I smoked at least a pack a day. I was an active duty soldier and smoked even heaver during my two deployments. I knew I had to quit and had tried multiple methods. Cold turkey never worked and I would be back on the smokes within a few days. The patches and gums made me sick, so again, back on the smokes. My experience with Chantix was the worst though, and almost cost me my life.
I had finally decided to talk to my doctor about quitting. I had tried all the over the counter methods with no success and had seen an ad on TV for Chantix. I asked my doctor and he was all too quick to prescribe me this new miracle drug in the fight against tobacco. I picked up my first month’s supply and was eager to get started. The first week was interesting to say the least. I had the most vivid nightmares I had ever experienced. Every night I dreaded going to sleep because I never knew what horrors awaited me. And for someone who already suffered from PTSD due to deployments, it just made the situation even worse. But I soldiered on, this was going to help me quit right? I stopped smoking as instructed at the start of the second week.
The nightmares continued, but the meds were doing their job so I kept taking them. Near the end of the third week, my wife and two young children went out of town for the weekend to visit family. I was on call for the Army and wasn’t allowed to travel more than 50 miles from the base, so they left me at the house. Saturday evening, I was watching TV when all of a sudden I decided that I should end my life. Just like that. No hesitation, no questioning, it was as routine as going to the kitchen and making a sandwich. I went to the kitchen, grabbed a bottle of sleeping pills that I was prescribed for my PTSD, downed about 20 of them, and went back to watching TV. If my wife hadn’t decided to cut their trip short and come home that night, I wouldn’t be here writing this message to you now.
I woke up in the ER surrounded by my family. My wife arrived to find me unconscious and unresponsive on the sofa with the bottle of pills on the counter and called an ambulance. I had never had a history of depression, nor ever had a suicidal thought. But on this miracle medication, I came within moments of ending my life. My children watched as I was loaded on that ambulance near death. My oldest still vividly remembers that night!! Needless to say, I threw out the Chantix and never looked back. Within three days, I was smoking again.
Around 2011, during a deployment to Afghanistan, I purchased my first electronic cigarette online. It was one of the ones that look like a cigarette with a light on the end of them. You can find them in most gas stations now. It was a cheap one made and shipped from China, but I was intrigued by this device and loved being able to intake nicotine without having to go out in the 120 degree heat. It wasn’t meant to replace smoking all together, just supplement it. When I got home later that year, I threw the cig-alike out and returned to my old habit full time.
Winter was always the worst for me because of all the damage done over the years from smoking. Any time I got sick, it seems it lingered with me three times longer than anyone else. I decided I wanted to try vaping again in winter 2012 when a friend of the family had opened up a vape shop. I purchased a newer ego setup with a refillable Kanger Protank. This almost did it for me a few times, but it just wasn’t enough for me to quit. I’d do well for a week or two, but then I’d get stressed and buy a pack of smokes.
Finally, in winter of 2015, I came down with Bronchitis, close to full blown pneumonia. I couldn’t breathe, let alone smoke due to the burning in my chest. I picked up my e-cig again and just like in Afghanistan, I was able to use it to supplement for my nicotine fix. Anytime either I or my wife get sick, we are banished down to the basement so as to not get everyone else in the house sick. It sounds cruel, but we’ve got a full sectional sofa, a plasma TV with surround sound, cable, and a PlayStation down there, so it’s not the worst place to be on the mend. In my isolation, I started looking more into vaping and discovered a series of videos from a rather large Italian. After watching a ton of these videos, I knew what I needed to do.
Once I was able to leave the house again, I went to my local vape shop and bought an iStick 50w, a Kanger Subtank Mini, and a vanilla custard flavored liquid at 12mg nic. Of course my wife wasn’t happy when she saw the price of my new hobby. Within a week of that purchase, I was able to put down the smokes for good. This was March 7th, 2015 and I haven’t had a cigarette since. The secret for me was a combination of the right flavor, the right nicotine, and a device capable of a direct lung hit. See, I didn’t need something to replicate a cigarette, like a mouth to lung device, because that just made me want an actual cigarette. What I needed was something new. Something different. The Subtank was that device for me. Don’t get me wrong, I have no issue with those who enjoy a MTL vape, it just didn’t work for me.
Since I started vaping full time, I have noticed a major difference in my day to day life. I can climb up a flight of stairs without losing my breath. I can play and keep up with my now three children, the third of which wouldn’t be here if I had been successful in taking my own life. I’ve even started running again, something I haven’t been able to do in many years. I started vaping at 24mg of nicotine, near the top available, and I’m now down to 3mg. Thank you Phil for opening my eyes to what is truly possible. Vaping has changed and even saved my life!!
NEW IN THE QUEUE – THE SX MINI Q CLASS BY YIHI – 6/8/16
The Q-Class just showed up today. BTW – What’s Joule? 🙂
Although it doesn’t feel bad, it doesn’t have that ROCK SOLID feel of the M or ML class.
Here’s some additional information from Yihi (don’t blame me for the misspellings):
SXmini Q Class
1. Dual 18650 battery device.
2. Maximum 200w.
3. Powered by new YiHi chip SX450.
4. Alloy Zinc+Stainless Steel+Maginet.
5. Replaceable Cover.
6. Balance charge onboard, charge for 2 batteries at the same time.
7. Custom your own Logo&Menu.
8. Support all TC wire SS/Ti/Ni… to 0.0001ohm coil resistance.
9. Intelligent taste control systerm SXi-Q.
Your taste visiable&customizable.
Powerful+/Powerful/Standard/Soft/Eco can be understood as AT(Automatic Transmission) when driving the Car. S1,S2,S3,S4,S5 can be understood as MT (Manual Transmission) ………
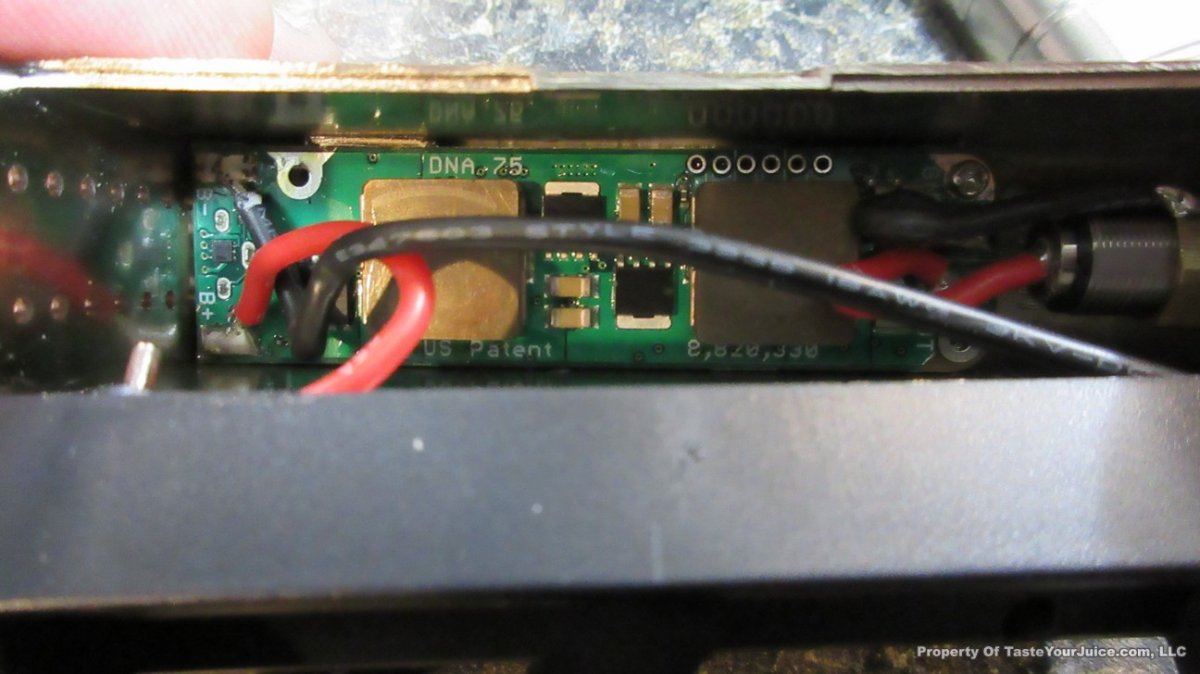
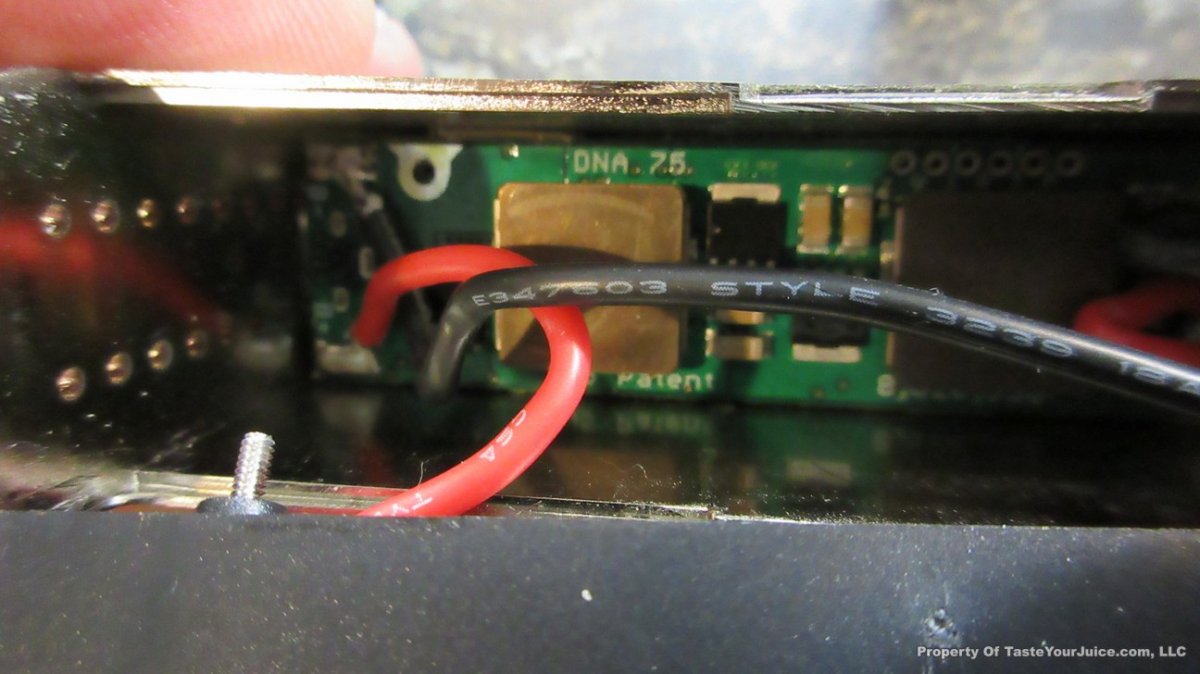
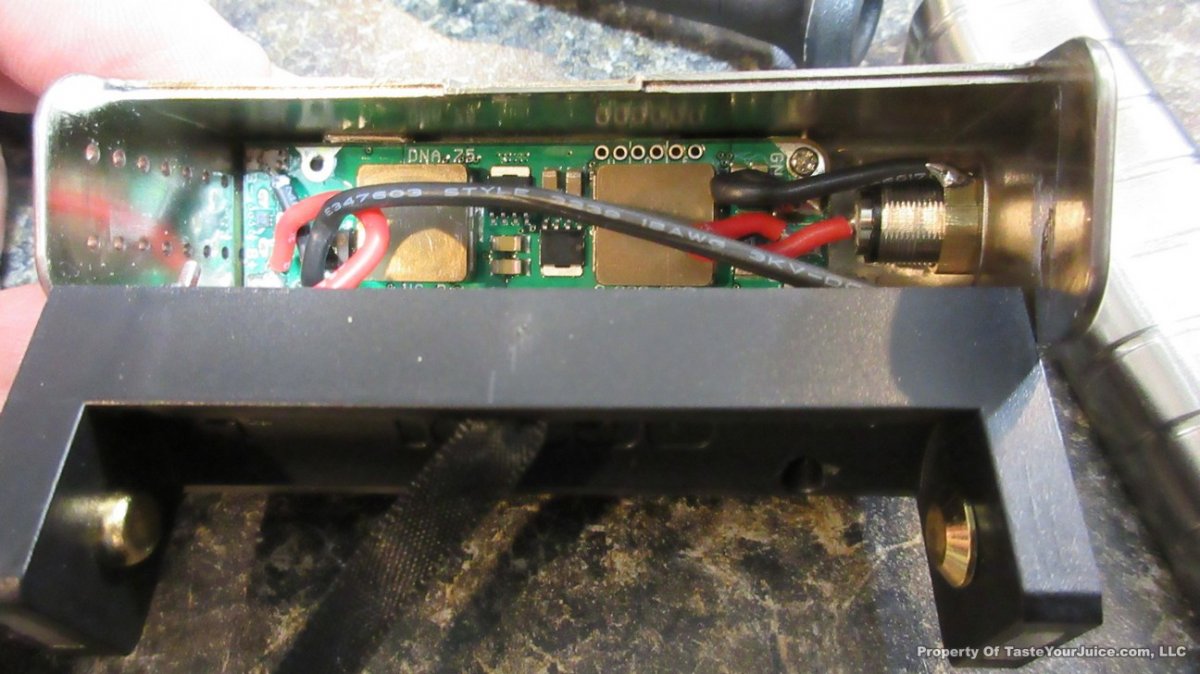
NEW IN THE QUEUE – THE SMY SDNA 75 – 6/8/16
Another DNA-75 box has shown up, and I’m quite sure it won’t be the last… this time from SMY.
I’m actually looking forward to seeing the DNA-75 making it’s way into some high end stab wood mods. Although I like the Yihi boards, I do prefer the TC signal of the DNA boards.
With this one… nothing too fancy, but it looks like Daniel of DJLsb Vapes is involved, so that makes me feel pretty confident that the device is set up properly.
Here’s some additional information from SMY:
After SDNA 200, we will bring out SDNA 75 box mod with the new DNA75 chip from Evolv. On the one hand, we will keep going with the advantages of SDNA 200, use stainless steel fat daddy style 510 connector, make it compact size with single 18650 removable battery, most importantly, we will continue work with DJLsb Vapes Daniel, he will test SDNA 75 and help with the pre-programmed firmwares,which can make the SDNA 75 have all the TCR presettings right out of the package box, super easy to use, plus the ergonomical appearance design, accurate temperature and stable performance from DNA75 chip, SDNA 75 is going to be a very special, fully-functional and convenient high end hardware for you.
Powered by the new DNA75 chip
> Single 18650 battery
> Ergonomically comfortable hand feeling
> Small size: 44mm* 23mm* 84mm
> 4 colors: Black, red, silver, wood
NEW IN THE QUEUE – THE KANGER CUPTI – 6/8/16
I took a quick look at this and all I could think of was… “my, what a large shaft you have”. 🙂
Once thing is for sure, the air as a LONG way to go before it gets to your mouth!
Is this Kanger’s answer to the new Joyetech eGrip or perhaps their answer to the leaky Neebox?
Hmmm… a side by side review with this and the eGrip II. That may be interesting.
This is another closed bottom design where the air comes in at the mouthpiece, down to the coil, and back up the (rather long) air shaft. With this design, the Neebox Leakiness is no longer a concern.
Here’s some more information from Kanger:
1. 5.0ML
2. Leak-free
3. 75W output
4. For MTL and DL
5. All in one device
6. CLRBA(optional)
7. Replaceable 18650
8. Temperature Control
9. Replaceable Pyrex glass
10. Durable surface furinshing
I think they meant “finish” and WHAT? No included RBA? That’s not like you Kanger!
NEW IN THE QUEUE – THE VAPORESSO NALU RDA – 6/8/16
I’m probably not going to review this one as I don’t look at very many drippers, but I figured I’d at least share some information and photos. If you’re into 24mm drippers, with large posts and holes for larger builds, big air, and big liquid/drip reserve, this one may be for you…
From Vaporesso:
Nalu RDA by Vaporesso is designed for vapers, by vapers. Renventing the way to convey power, here at Vaporesso we have created the ultimate RDA. Nalu means wave in Hawaiian, just like the ripples of the pacific ocean this RDA is untamable. Boasting two giant posts to fit your demanding builds and a chamber offering a peek to your single or double coil builds, you will no longer have to keep count of your hits before the next drip. The bottom airflow system will guarantee the most vapor and the best taste of your juice and with the sharply improved connective pin, we guarantee the most effective and precise resistance control.
Features:
Supporting single coil build with independent airflow, stable and strong
Bigger post holes to accommodate larger gauge
Massive drip reserve
No dry hits thanks to the chamber windowsSpecifications:
Diameter: 24mm
Length:39mm
Weight:60gIn The Box:
1 Nalu RDA
1 Dual Coils Assembly
1 Allen Key
1 Squonking/ Bottom Feeder Pin
2 Drip tip
4 Spare Screw
4 Spare Ring









































































































































































































































































 Store
Store












