Author: pbusardo
CHINA TRIP 2018!
A PBusardo Video – China Trip 2018!
In this video Dimitris and I take you back to China! We see product assembly, packaging, and clean room coil assembly at the Innokin facilities and have some fun along the way. We hope you enjoy it!!
The Links:
Past China Videos:
Video 1 – 2015 – Innokin
Video 2 – 2015 – Kanger
Video 3 – 2015 – Aspire
Video 4 – 2015 – Boomertech & The Clone Factory
Video 5 – 2016 – Joyetech
Video 6 – 2016 – Vaporesso
Video 7 – 2016 – Yongdeli Battery Factory
Video 8 – 2017 – SEVIA USA, Innokin, Smok Offices & Dan 🙂
The Video:
*NOTE: Any use of these videos in part or in their entirety without Phil Busardo’s expressed written consent is strictly prohibited.
The Photos:
THE INNOKIN EQ
A PBusardo Review – The Innokin EQ
In this video we take a look at the Innokin EQ. A satisfying little pod system for both salt AND standard nic!
We also find out who won the last contest & kick off a new one.
The Links:
Innokin
My Vapor Store
Vaporesso
The Video:
*NOTE: Any use of these videos in part or in their entirety without Phil Busardo’s expressed written consent is strictly prohibited.
The Photos:
MY NEXT EVENT – Vapexpro Athens 2018
THE SMOKER’S SHOW – EPISODE #12
The Smoker’s Show Episode #12
In this episode, we…
- talk about the China trip and take you to the Innokin factory in Shenzhen China to show you device assembly, coil assembly, and packaging.
- talk about the Juul lawsuit.
- do some unboxings and feature tours of the Innokin EQ and the Joyetech Teros.
We also take your calls and questions!
The Smoker’s Show is a vape show not for vapers, but for smokers.
A show to get information about vaping, to debunk vaping myths, to discuss vaping terminology & technology, and to look at and review starter kits for the transitioning smoker.
We urge all vapers to invite those they know who still smoke to watch the show!
Thank you all for your support and let’s convert more smokers together!
Thank you to and please support our Sponsors!
*NOTE: Any use of these videos in part or in their entirety without Phil Busardo’s and Dimitris Agrafiotis’s expressed written consent is strictly prohibited.
See all the Episodes HERE.
Materials referenced in this show:

THE SMOKER’S SHOW TONIGHT AT 9PM EST
THE SMOKER’S SHOW RETURNS TOMORROW NIGHT!
VTA FILES FULL SCIENTIFIC DEFENSE OF FLAVORS

VTA SUBMITS FULL SCIENTIFIC DEFENSE OF FLAVORED VAPOR
IN RESPONSE TO FDA ANPRM
July 21, 2018 – On July 19, 2018, the Vapor Technology Association submitted VTA’s Comments on the FDA’s Advanced Notice of Proposed Rulemaking on Flavors, Docket No. 2017-N-6565. VTA put itself in a position to present to FDA a scientific defense of flavors in ENDS products after engaging scientific experts to review, assess, and analyze all of the peer-reviewed scientific research on flavors and vapor products.
This intensive analysis – which had to be completed in very short order – required a significant amount of resources, a decision that was made by VTA’s Board of Directors which understood the importance of making the strongest possible case based on science to defend flavors in vapor products. VTA wants to thank its regulatory counsel, Eric Heyer, Thompson Hine, LLC for his and the firm’s extensive work on VTA’s comments to the flavor ANPRM. The professionalism, responsiveness, and attention to detail by counsel enabled us to present a thoughtful, clear, balanced, and forceful defense of flavors in vapor products.
SUMMARY OF COMMENTS
First, VTA framed the issues for FDA’s analysis explaining that the uniqueness of ENDS products in the overall discussion of this ANPRM requires that FDA examine vapor products differently and impose on itself a higher standard of scientific certainty before taking any action to regulate flavored vapor. As part of that analysis, VTA reviewed the well-known conclusions about the relative safety of vapor products, the unique position that vapor products occupy on the risk continuum, and the unique attributes of vapor products that distinguish them – and any policy related to flavors – from combustible tobacco products.
Second, VTA presented the scientifically based arguments for how and why flavors are helping adult smokers reduce and quit smoking combustible cigarettes. VTA presented the key studies and surveys demonstrating that flavors are a key factor in cessation and, as importantly, VTA demonstrated the many scientific failings of the only studies that try to deny that flavors assist with cessation, explaining to FDA why those studies can carry no scientific weight.
Third, VTA presented the scientifically based arguments for why the concern of a “gateway” to cigarette smoking is entirely misinformed – emphasizing that there is no reliable science which could justify limiting flavors because of youth or adult initiation.
Fourth, VTA explained that the existing toxicological evidence on flavors and vapor products is simply underdeveloped and does not provide a basis for regulating flavors. This is especially true when FDA is required to balance the interests of the potential adverse consequences, such as the real likelihood of smokers relapsing, the continued sale of unregulated products on the black market or the rapid expansion of an unregulated DIY market.
Finally, VTA encouraged FDA to consider the fact that we have many tools that can be deployed and strengthened to continue the rapid decline in youth vaping and emphasized the importance of the VTA Marketing Standards being adopted to further that goal. In contrast, VTA noted that, other than vapor technologies, we simply do not have meaningful tools to help adult smokers quit, especially given the poor track record of existing NRTs that have had every marketing and regulatory advantage. In other words, FDA cannot take any precipitous action to limit flavors in vapor products since that would simply remove what is proving to be an important tool in the smoker’s arsenal for reducing or quitting cigarettes.
HIGHLIGHTS OF VTA QUOTES
AND CONCLUSIONS
A review of the Table of Contents provides a full overview of the arguments and conclusions presented by VTA, but here are a few highlights from the lengthy submission from VTA.
FDA HAS NO SCIENTIFIC BASIS TO LIMIT FLAVORED VAPOR; VAPOR UNIQUENESS DEMANDS SCIENTIFIC CERTAINTY BEFORE REGULATION
“Based on VTA’s review of the peer-reviewed research on the role of tobacco and non-tobacco flavors in ENDS, FDA does not have a sound scientific basis upon which to issue a product standard or otherwise restrict the sale or distribution of any ENDS flavor.”
“For the sake of both individual and public health, FDA must examine the role of flavors in ENDS products differently than any other product under consideration and why FDA should impose on itself the highest standard of scientific certainty before it acts to regulate or limit ENDS flavors in any way at this time.”
“In light of the predictable harms that would result to former, current, and future smokers if access to non-tobacco flavored ENDS were restricted, FDA should demand of itself the highest level of scientific evidence before considering potentially restricting access to ENDS products.”
“Because the proven health risks associated with ENDS products are so low and the potential benefits of such products are so high, FDA should demand of itself the most rigorous scientific standard of certainty before considering any product standard or other restriction on the sale of flavored ENDS products.”
FDA MUST EXAMINE AND TREAT VAPOR PRODUCTS DIFFERENTLY THAN COMBUSTIBLES
“FDA must resist the temptation to lump together ENDS products with combusted tobacco products since doing so serves no meaningful scientific or policy objective when evaluating completely different types of products – one, an organic agricultural product that is combusted, and the other a consumer electronic that delivers a vapor which contains zero tobacco – the only common attribute of which is nicotine.”
“In addition to taking into account the accepted scientific conclusions that vapor products are demonstrably safer than combustible cigarettes, FDA also must take into account the place that ENDS products occupy on the opposite end of the risk continuum from combustible cigarettes – a place occupied by the products that deliver nicotine without combustion and in the absence of any tobacco.”
“Although ENDS are encompassed in the Tobacco Control Act’s broad legal definition of “tobacco products,” they differ markedly from virtually every other product covered by that definition in multiple meaningful ways and so must also be treated differently as a matter of FDA policy.”
“It is clear that ENDS products cannot be viewed through the same policy prism as characterizing flavors in other tobacco products, including cigarettes.”
“If nothing else underscores the fundamental difference between ENDS products and tobacco products, it is the fact that the naturally occurring flavor of e-liquids prior to the introduction of flavorings is NOT tobacco because ENDS e-liquids do not contain tobacco. […] This distinction is important because many of the presumptions that FDA may have in connection with why flavors are added to combustible products do not apply to ENDS products.”
“As noted in various studies, and as is obvious from a cursory review of the marketplace, there is a wide selection of ENDS products on the market with varying levels of nicotine. This empowers the ENDS user with the ability to choose the amount of nicotine at which they start and, most importantly, choose lower levels of nicotine – including zero nicotine – as they mature in their use of ENDS. This fact makes ENDS products entirely unique from all of the other products subject to this ANPRM and again requires FDA to be circumspect about limiting its availability.”
FLAVORED VAPOR HELPS ADULTS QUIT
“[A] strong trend in the scientific literature supports the proposition that the availability of a wide variety of non-tobacco flavors in nicotine-containing e-liquids used in ENDS products further bolsters smoking cessation and promotes larger numbers of smokers to permanently transition to less harmful ENDS products. Rather than merely help sell more products, the availability of non-tobacco flavors in ENDS products actually advances the public health goals of reducing reliance on harmful combustible cigarettes and improving smoking cessation rates.”
“The existing reliable scientific literature on flavors and ENDS products-including longitudinal analyses, survey data, and experimental studies-trends strongly in favor of the conclusion that access to a wide variety of flavors-and particularly non-tobacco flavors-plays a critical role in encouraging cessation among existing smokers and preventing relapse.”
THERE IS NO GATEWAY TO SMOKING ASSOCIATED WITH FLAVORED VAPOR
“There is no reliable literature that concludes that the availability of non-tobacco flavors in ENDS products makes more likely any gateway effect of progression from ENDS to cigarettes. In the end, as Dr. Rigotti clarified from the NASEM Report, the “enormous amount of ecological data” makes it “hard to argue that there is a gateway there.”
“Any regulatory action that would restrict access to non-tobacco flavors on the basis that they attract youth to ENDS would be premature and any such action undertaken on the theory that such flavors promote a gateway effect to combustible cigarettes would be entirely without any scientific basis.”
“The predictable adverse public health effects of limiting access to non-tobacco-flavored ENDS products would far outweigh any speculative public health benefit. Consideration of the health effects associated with flavors in ENDS products also weighs against any product standard that would limit access to such products.”
ON BALANCE, FDA MUST PROTECT ENDS AND PROMOTE FLAVORED VAPOR
“The balancing of interests with respect to flavored ENDS products is relatively easy for FDA: FDA must prioritize helping the adult smoker desperately trying to switch to noncombusted products like ENDS. The short term individual benefits of ENDS have been recognized by NASEM, the relative safety when compared to deadly combustible cigarettes has been heralded by public health experts in the U.S. and around the world, and the potential long-term benefits are so critical to the public health of our nation that these considerations dramatically outweigh the speculative concern about initiation, no matter how much it may be sensationalized.”
“Never before has a revolutionary consumer technology offered an alternative pathway to cessation. […] Moreover, it is clear that ENDS products are so uniquely situated amongst all other “tobacco products” that FDA must recognize the ground-breaking tool that they offer FDA to achieve one of its biggest public health missions: eliminating cigarette smoking. With that goal at the forefront of all considerations, the balancing of interests in favor of ENDS products and flavors is easy.”
If you have any questions regarding the VTA’s defense of flavors, please feel free to reach out.
Thank you for all you do to defend vapor, and let’s fight this fight together!

Tony Abboud
Executive Director
Vapor Technology Association
NEW FROM REGULATOR WATCH – Flavor Fight | FDA Should Listen to People Not Bots
Hundreds of thousands of fake comment submissions have ground FDA’s regulatory process over the proposed regulation of flavors in vaping products to a halt.
As RegWatch first reported, a minimum of 250,000 bot submitted fake form-letters that are anti-flavors and anti-vaping in nature overwhelmed servers at Regulations.gov putting the entire public consultation process at risk. FDA could decide to throw out all submissions, including legitimate testimonials, provided by tens of thousands of vapers.
The vaping industry is fighting back. The Vapor Technology Association, a leading industry trade group, is running a coordinated public comment campaign to help vapers make an impactful “declaration” to the FDA on why flavors are essential to vaping.
In this extended edition of RegWatch, hear from VTA’s executive director Tony Abboud and learn what the strategy is going forward, to save flavors.
Only on RegWatch by RegulatorWatch.com
Produced by: Brent Stafford
Released: July 17, 2018
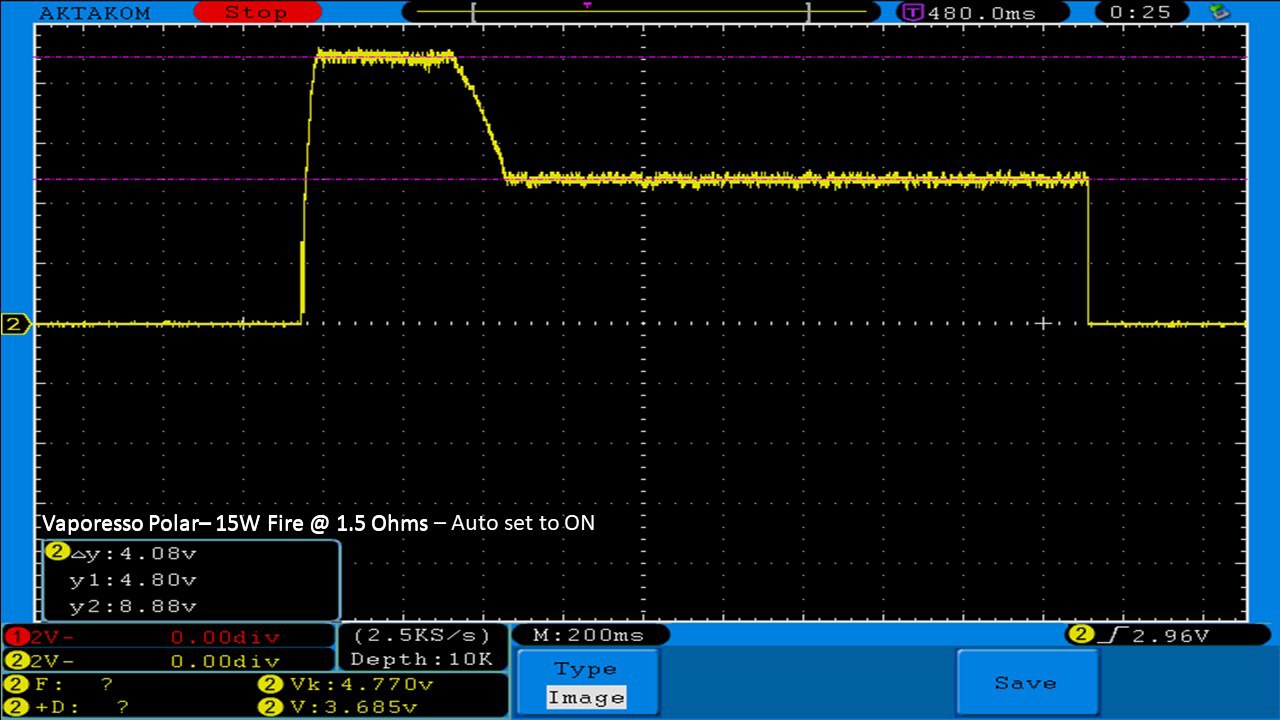
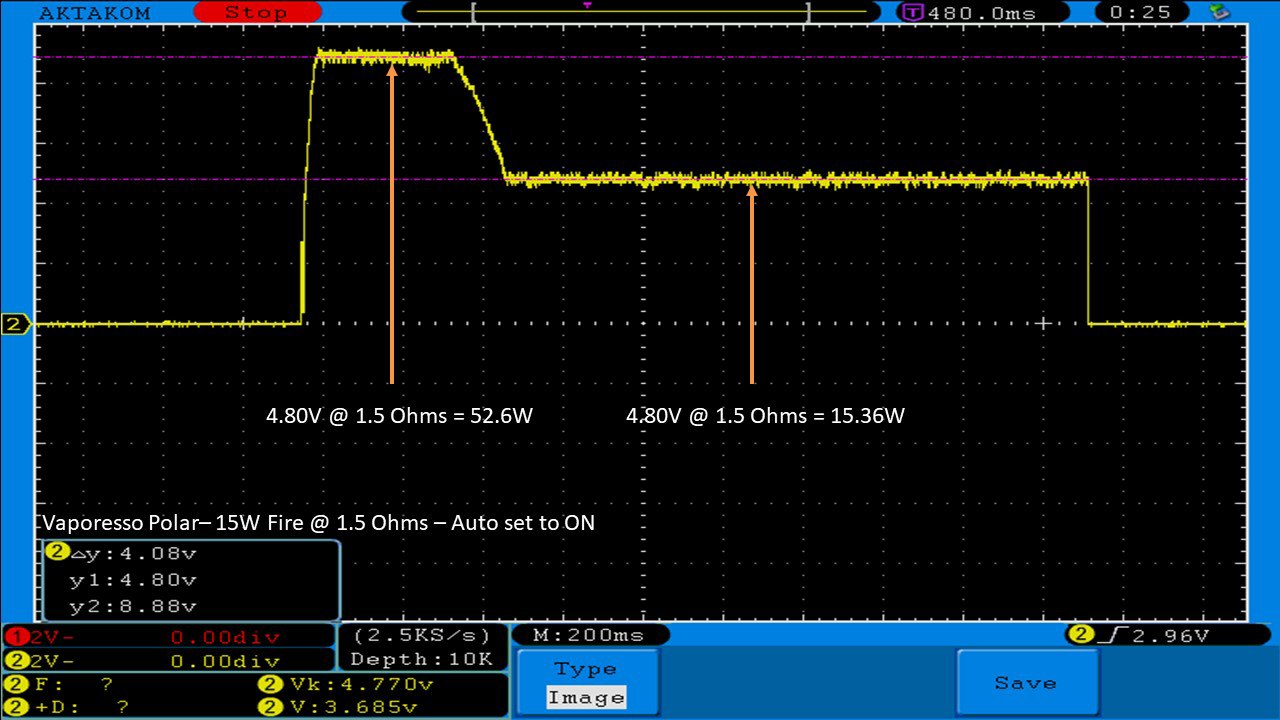
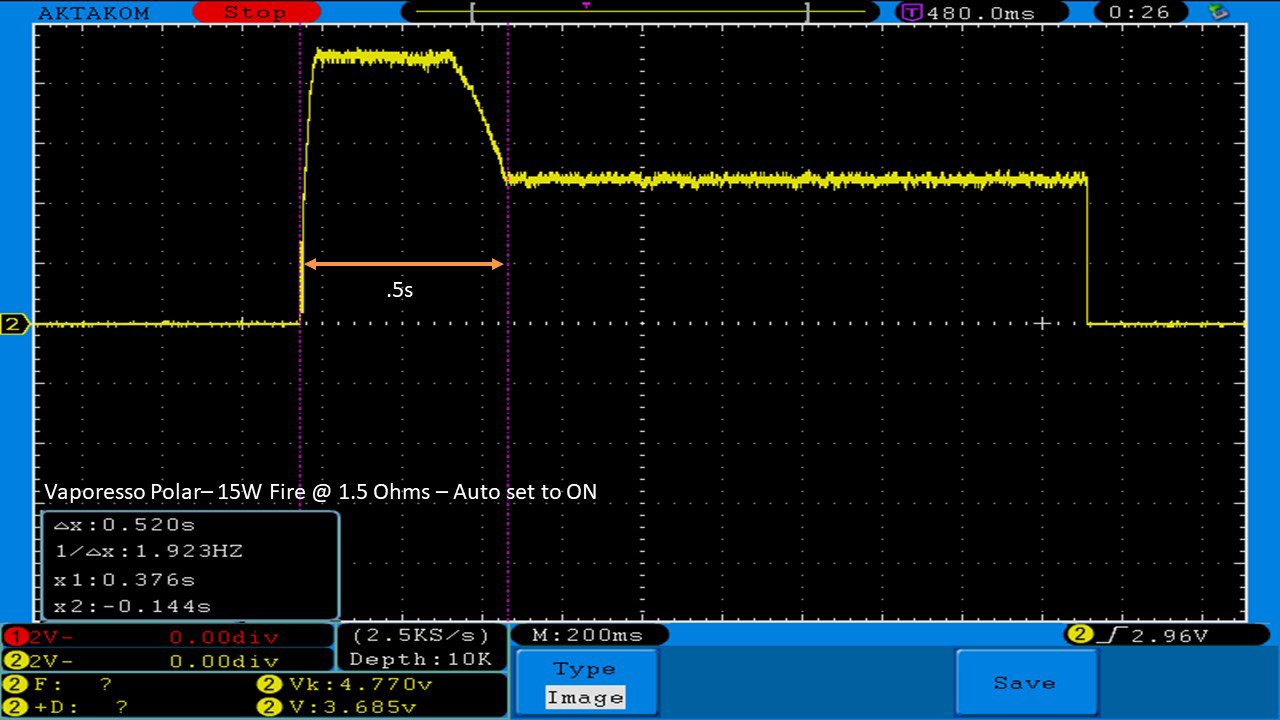
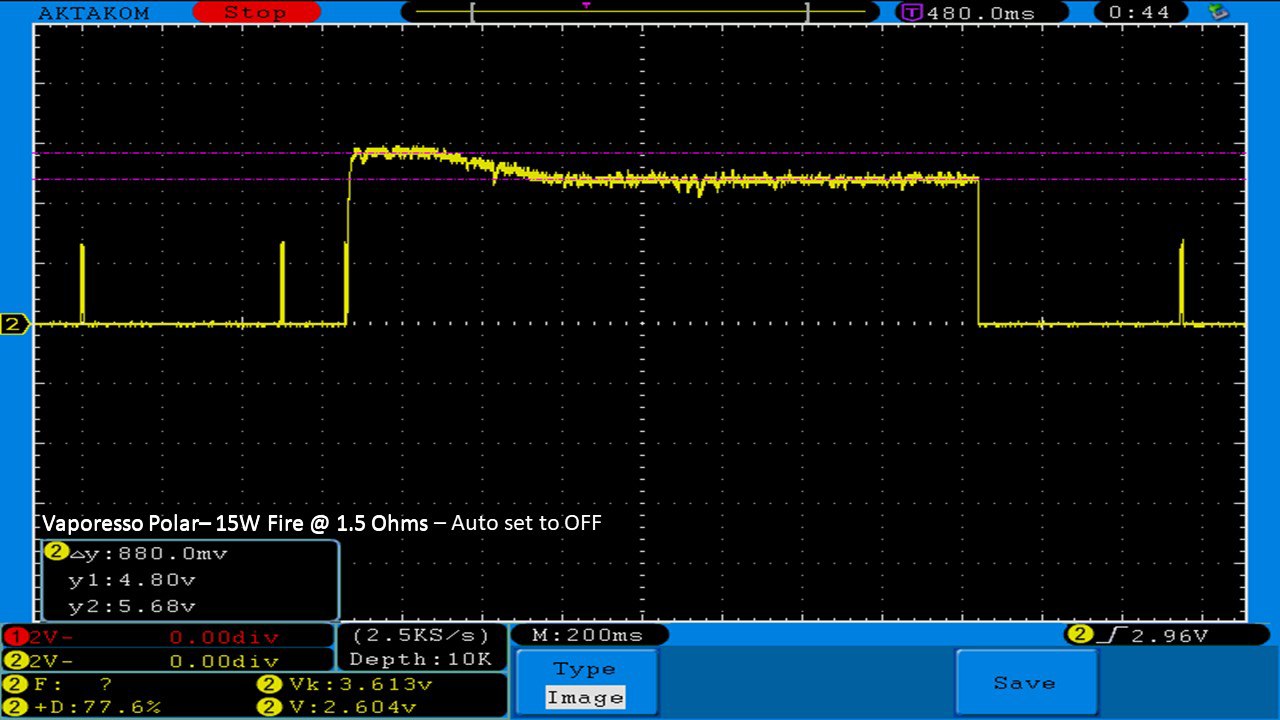
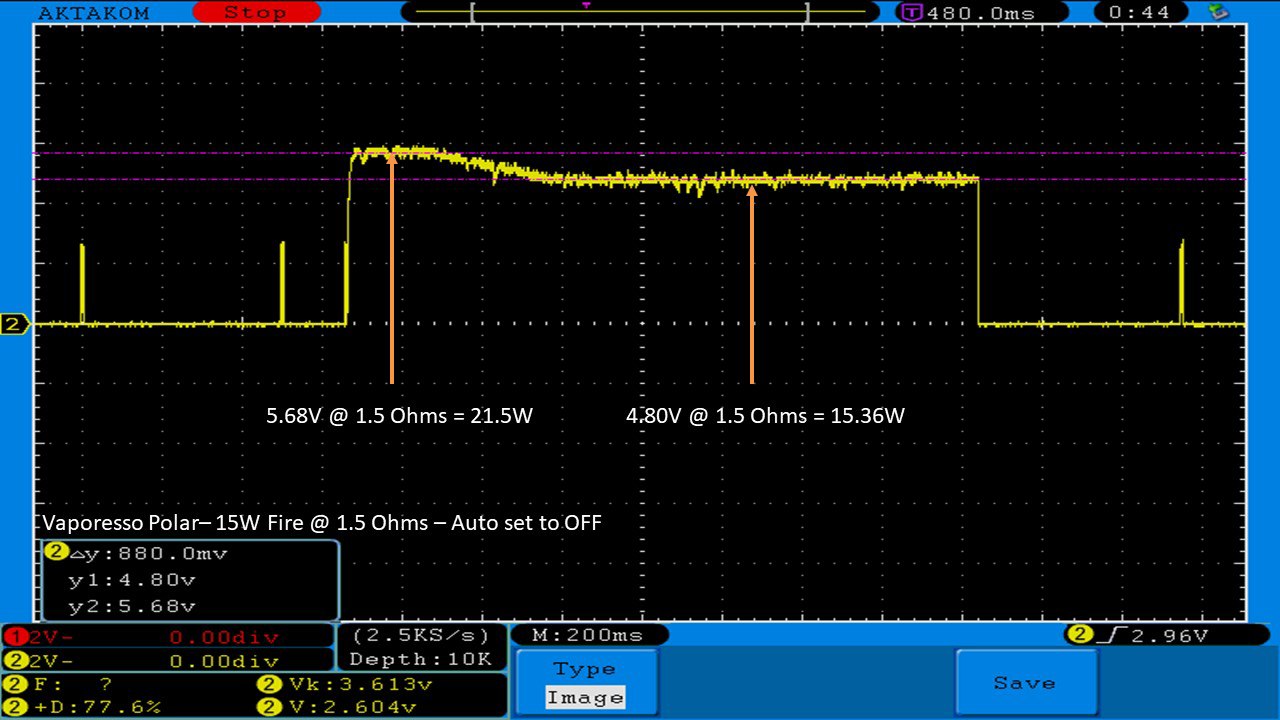
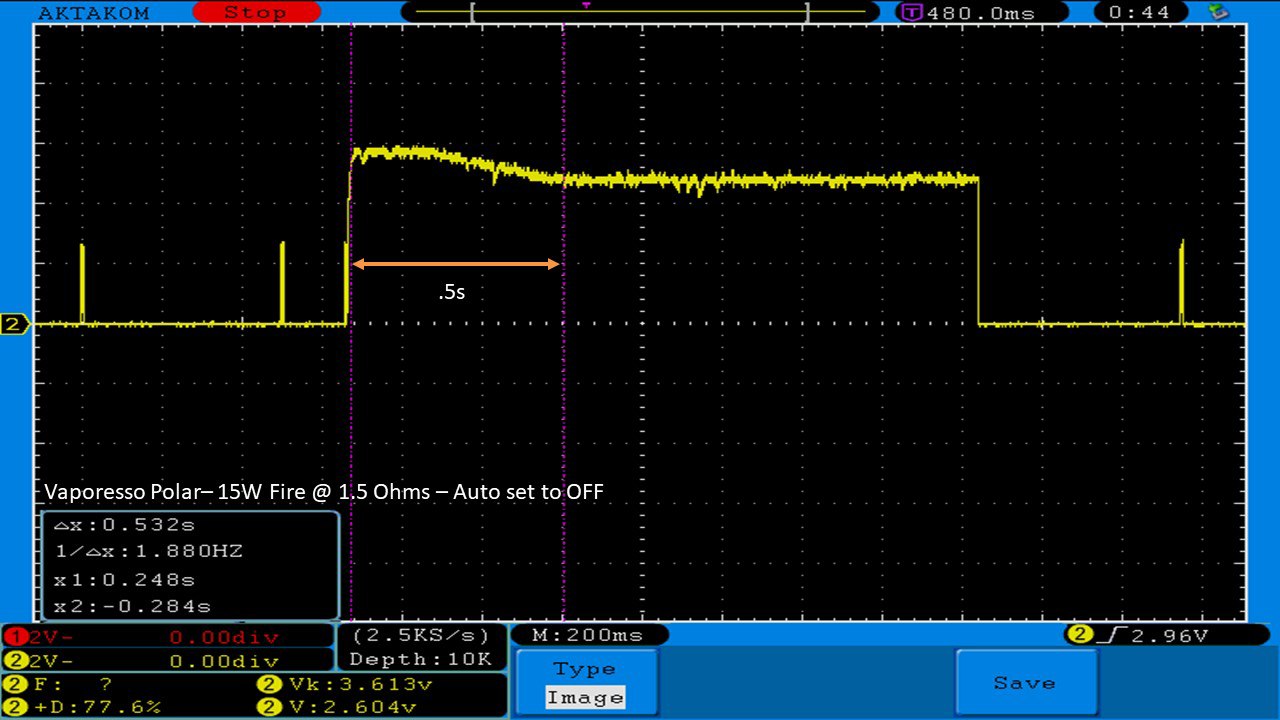
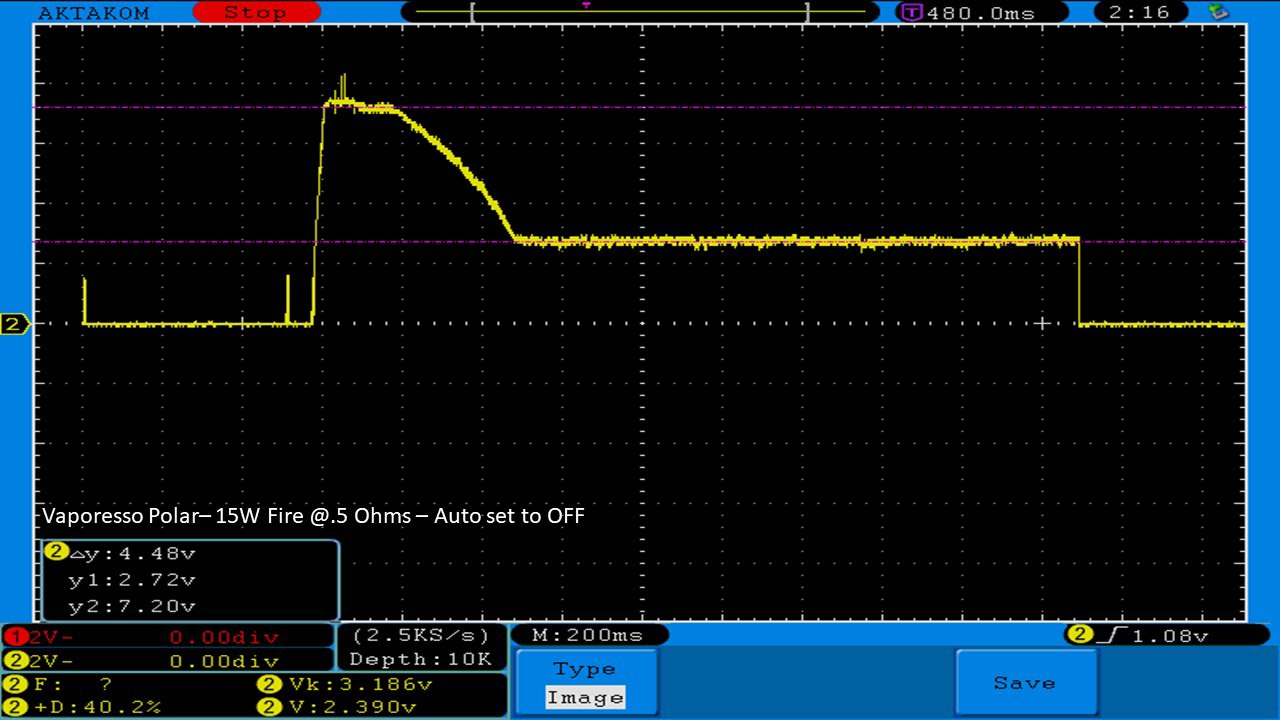
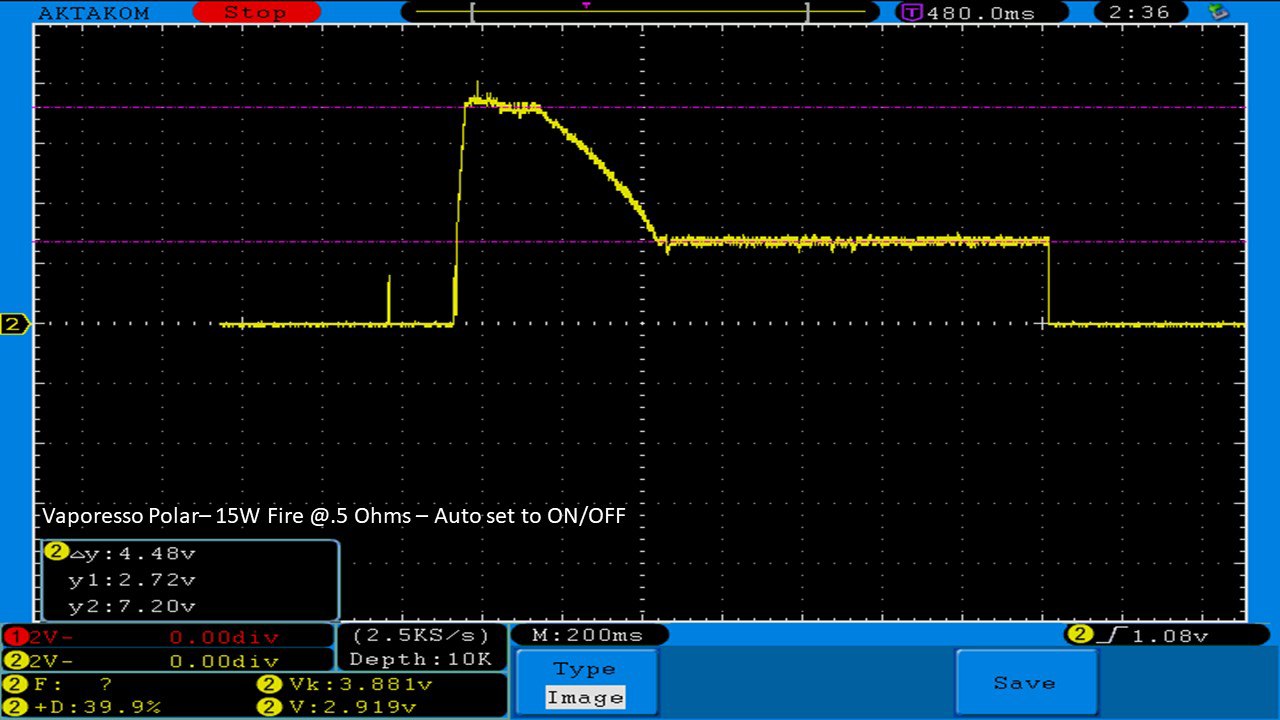
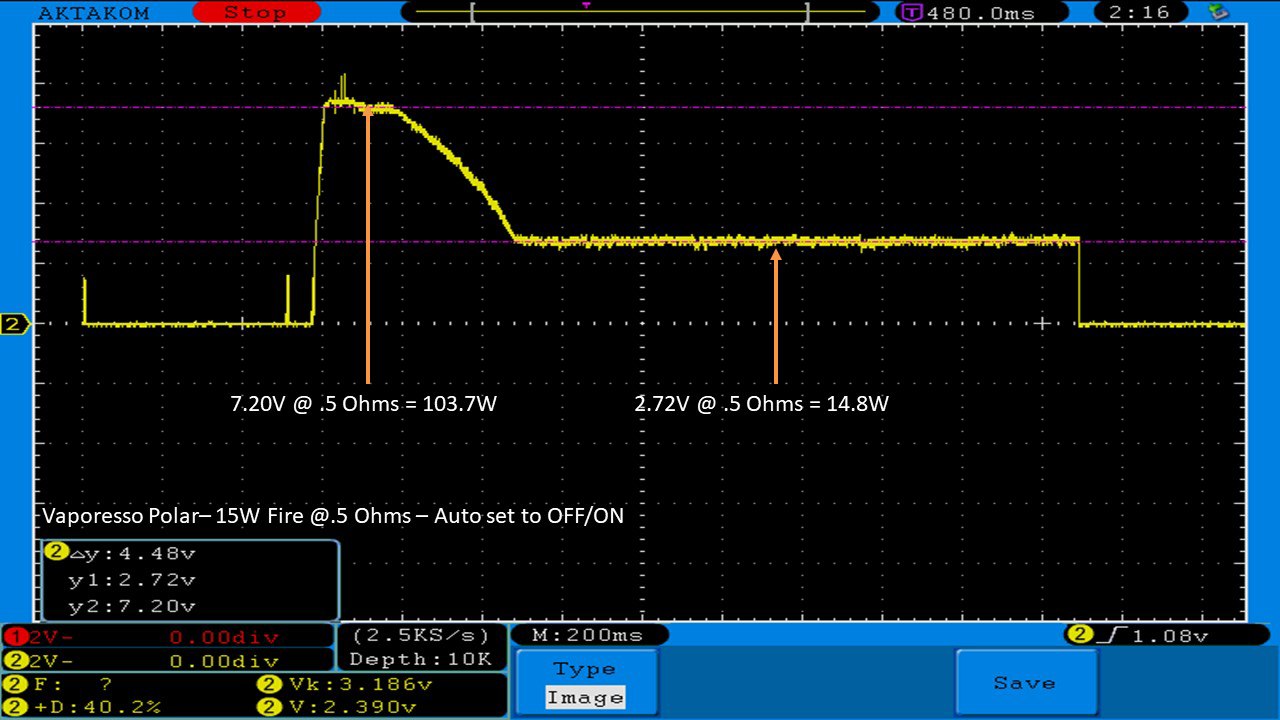
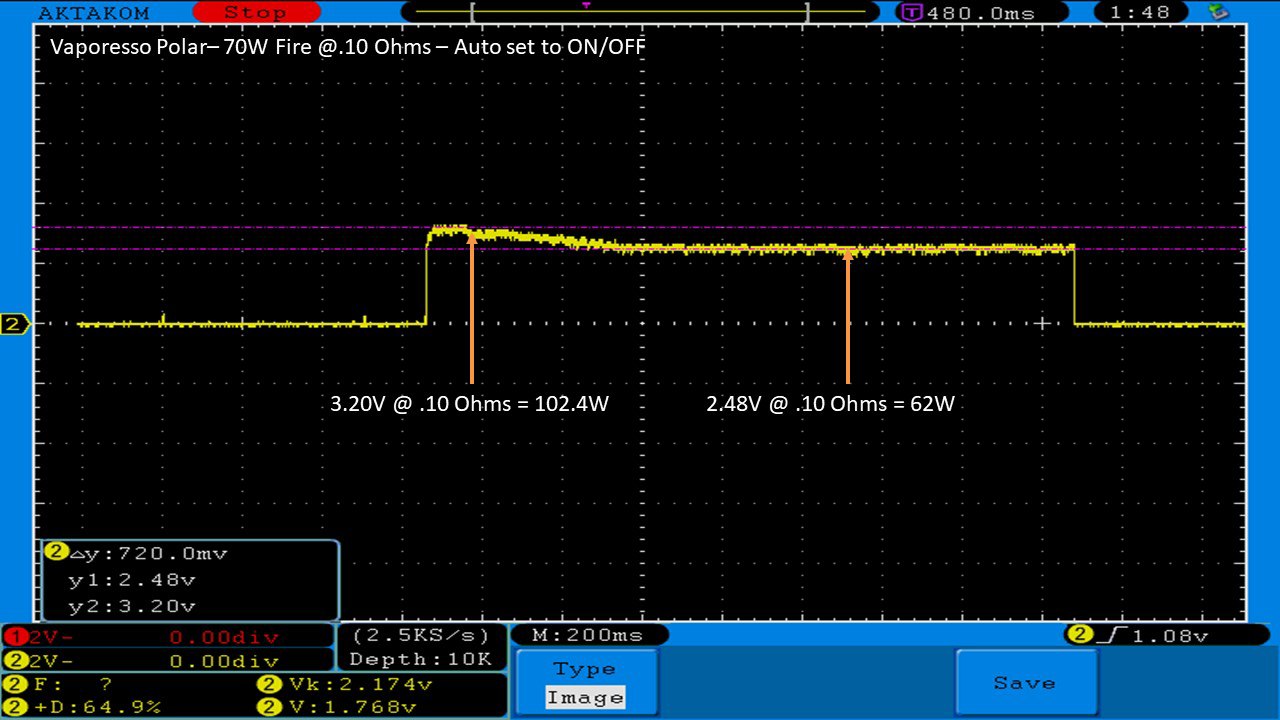
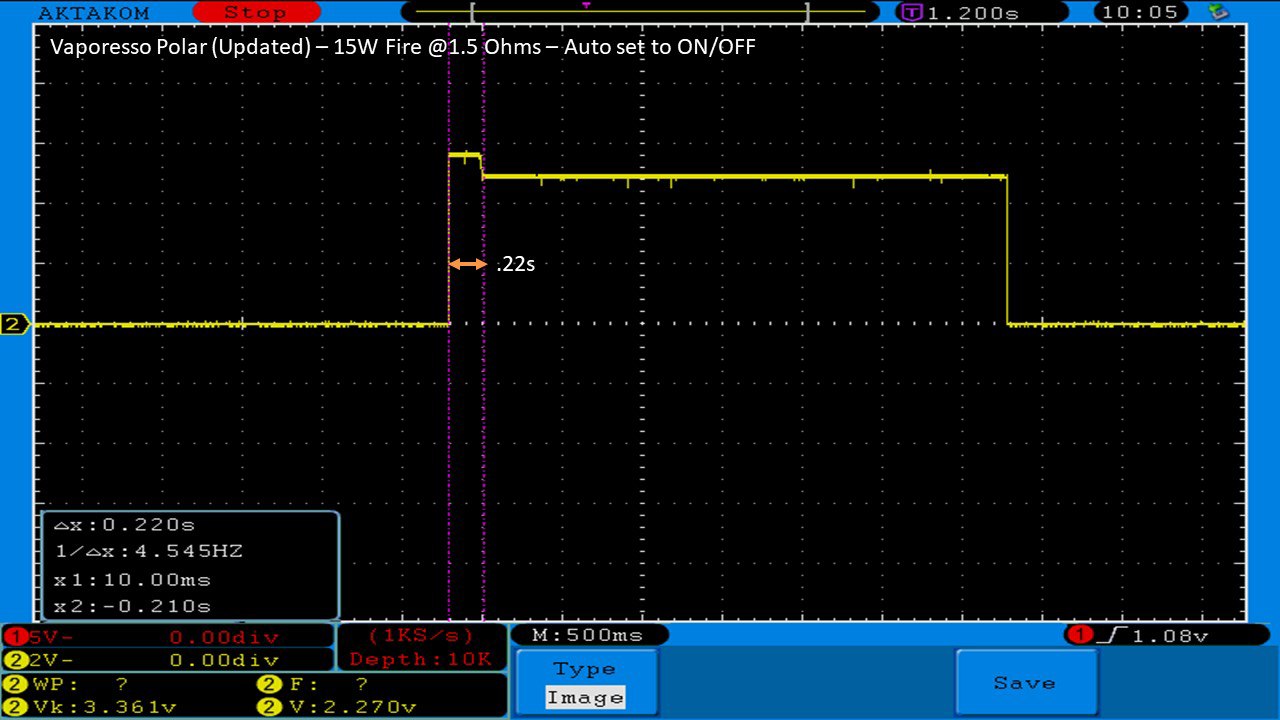
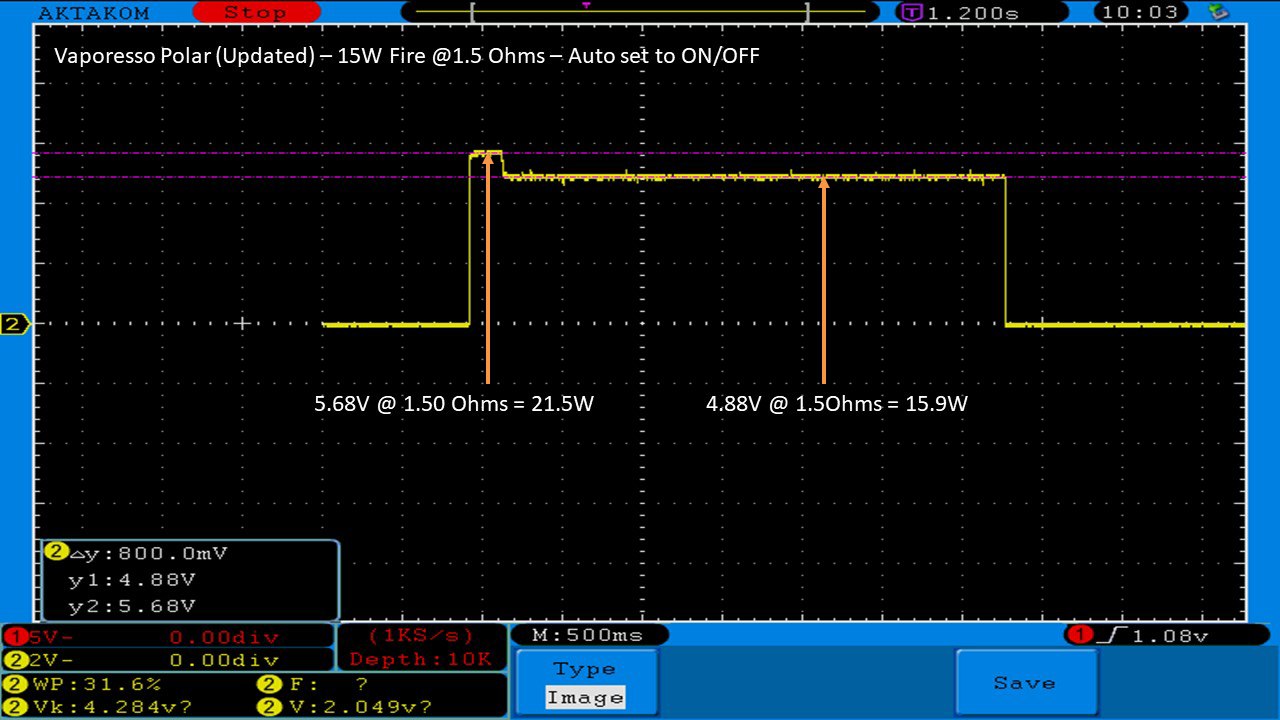
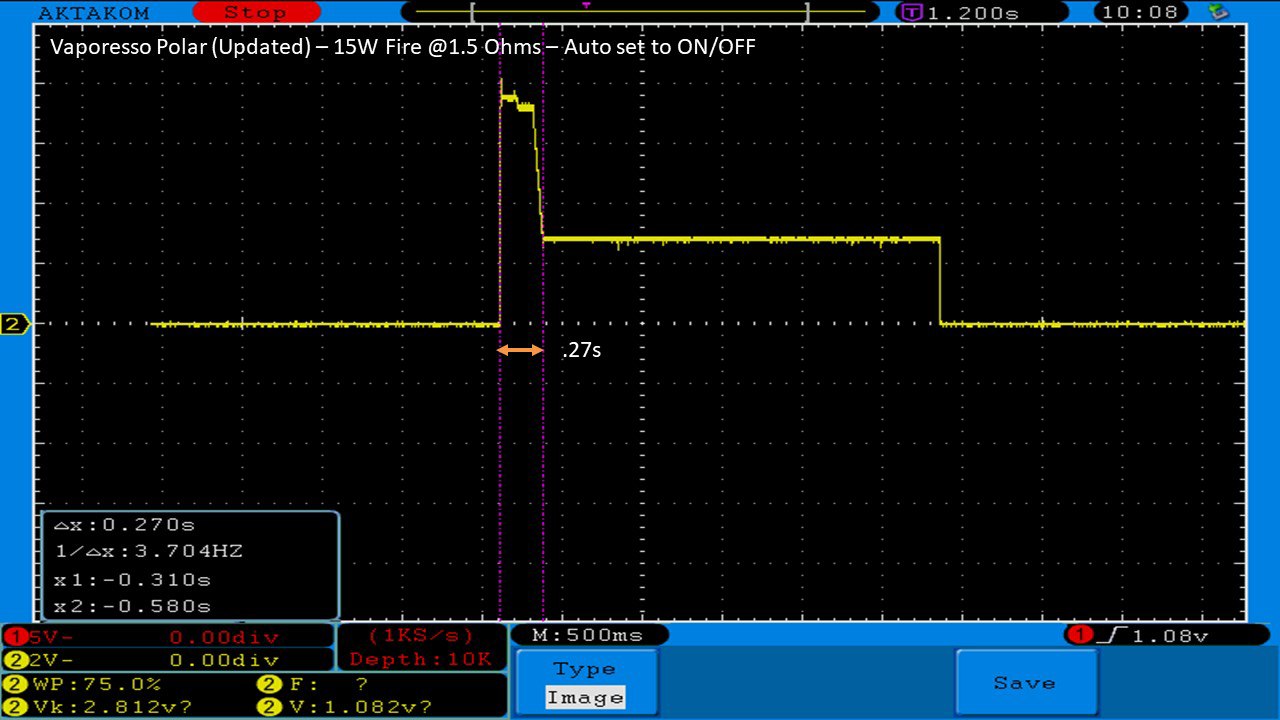
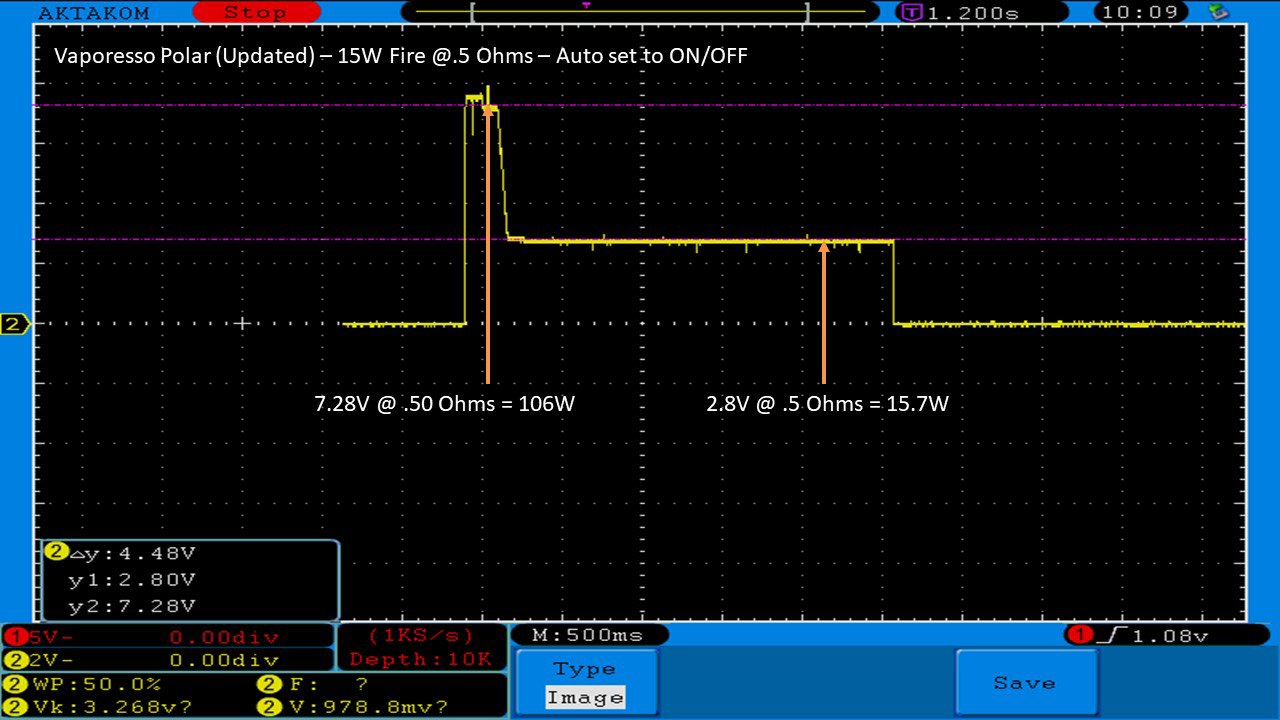
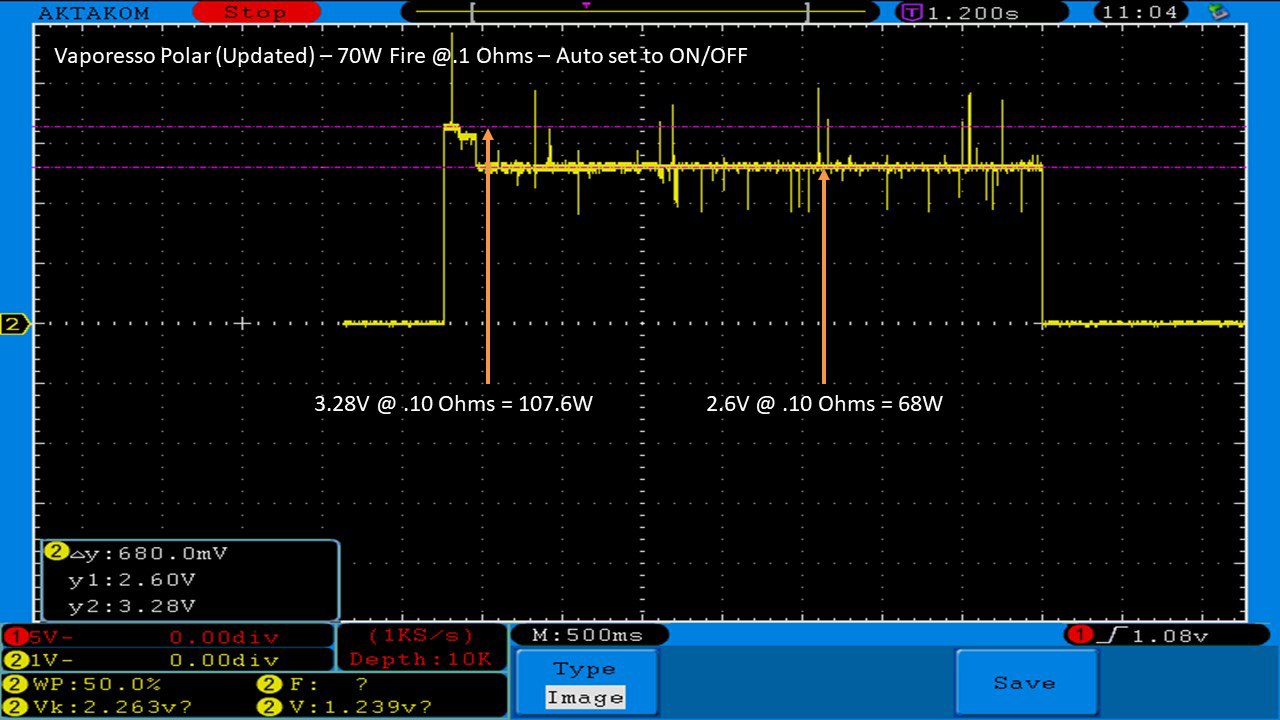
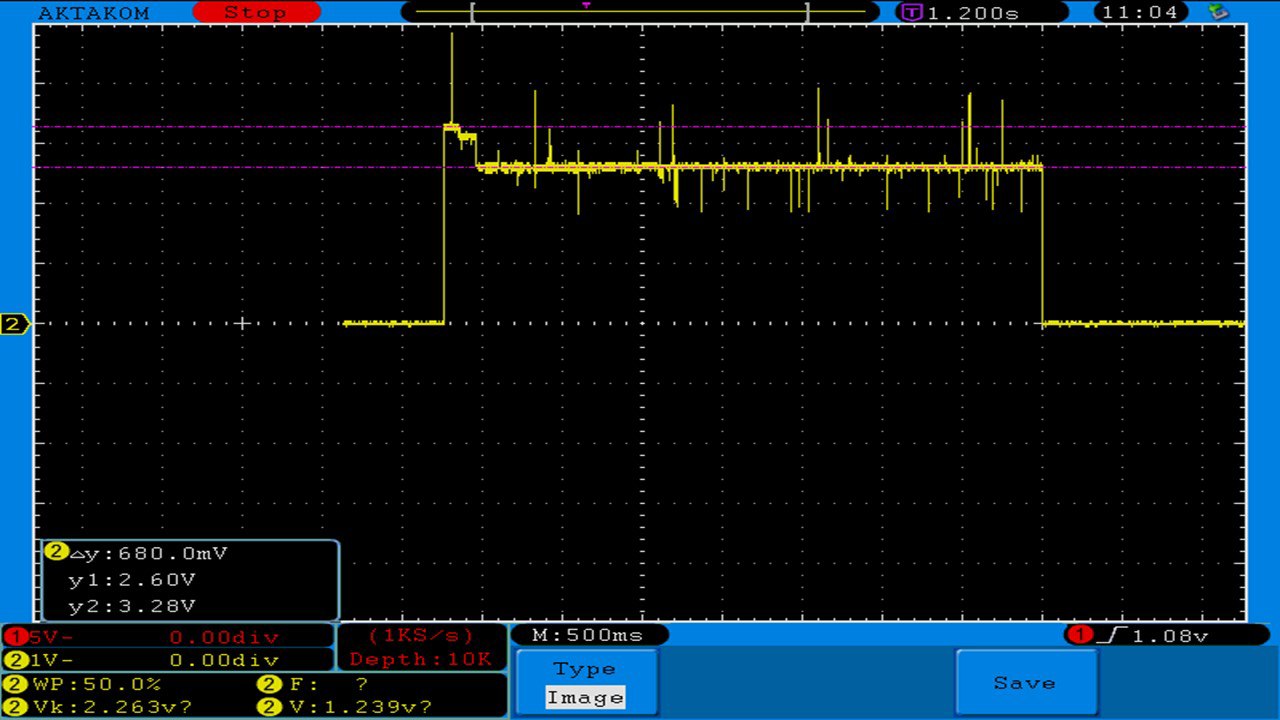
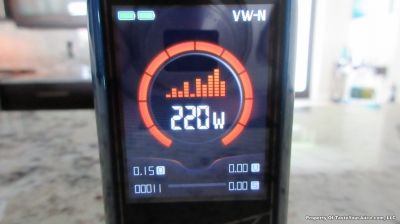
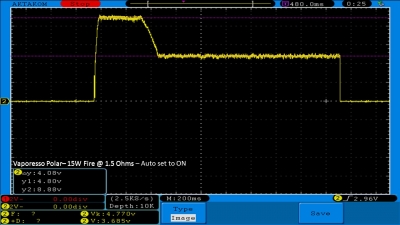
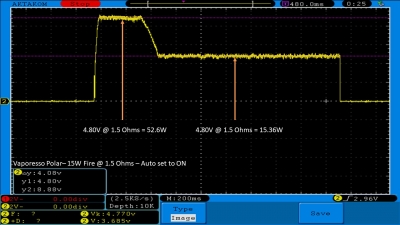
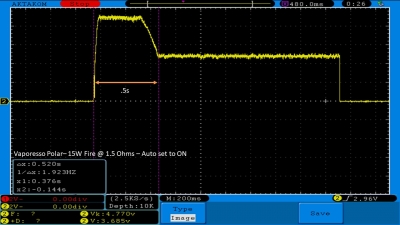
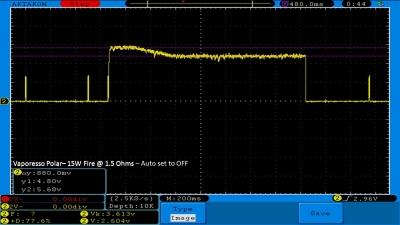
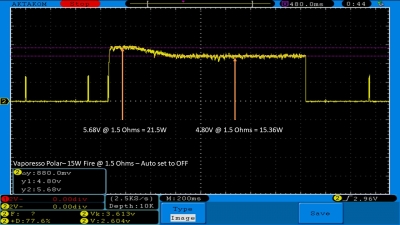
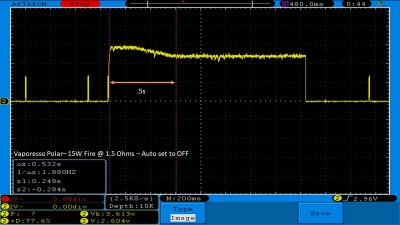
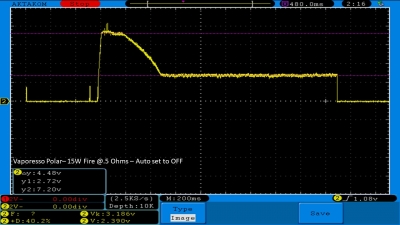
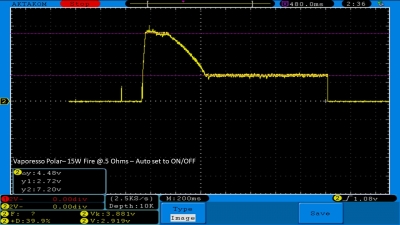
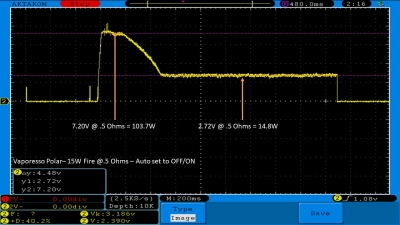
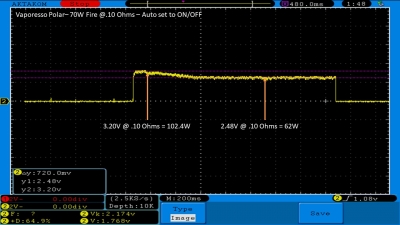
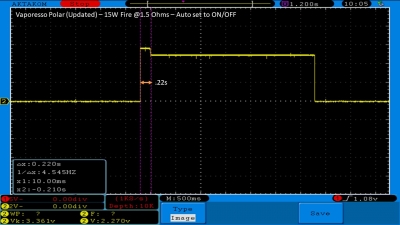
THE VAPORESSO POLAR (AGAIN) AND A NEW CONTEST!
A PBusardo Review – The Vaporesso Polar Kit (Again) + New Contest!
In this video we take another look at the Vaporesso Polar.
Did they fix the issues? Well… kinda. Take a look. This time it get’s the “Full Busardo”.
The Links:
The Video:
*NOTE: Any use of these videos in part or in their entirety without Phil Busardo’s expressed written consent is strictly prohibited.
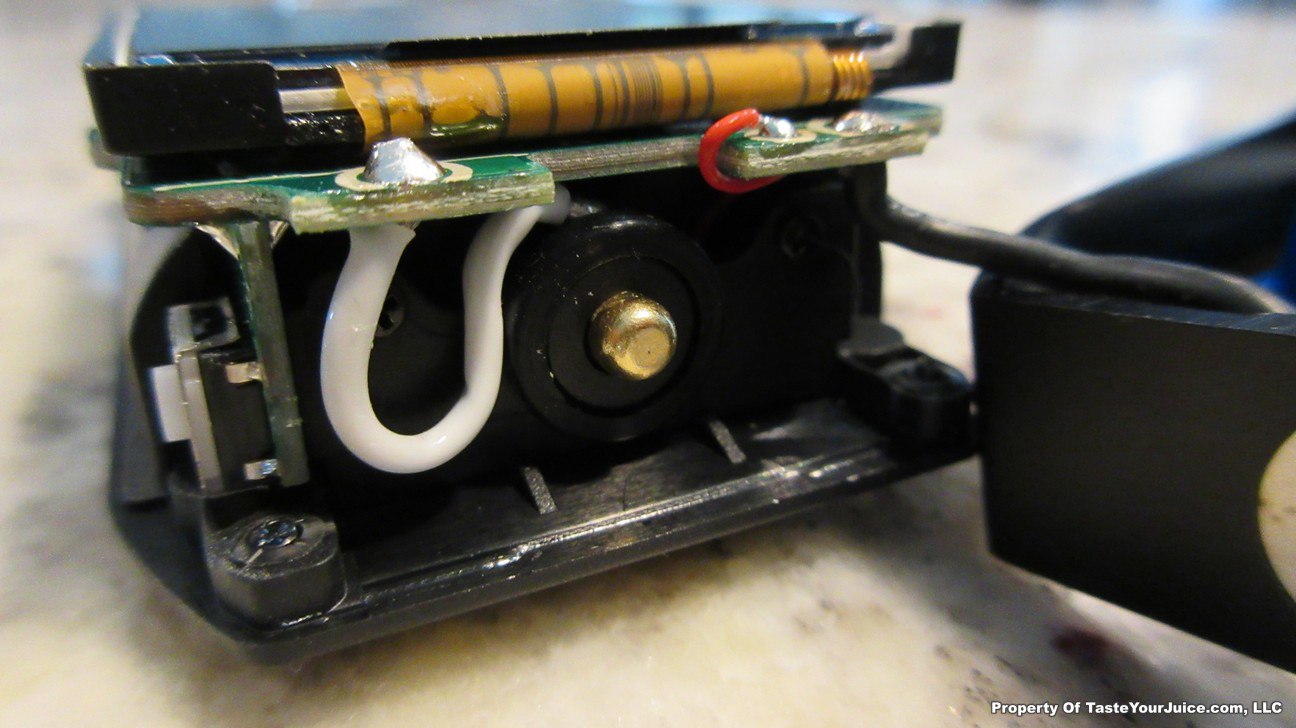
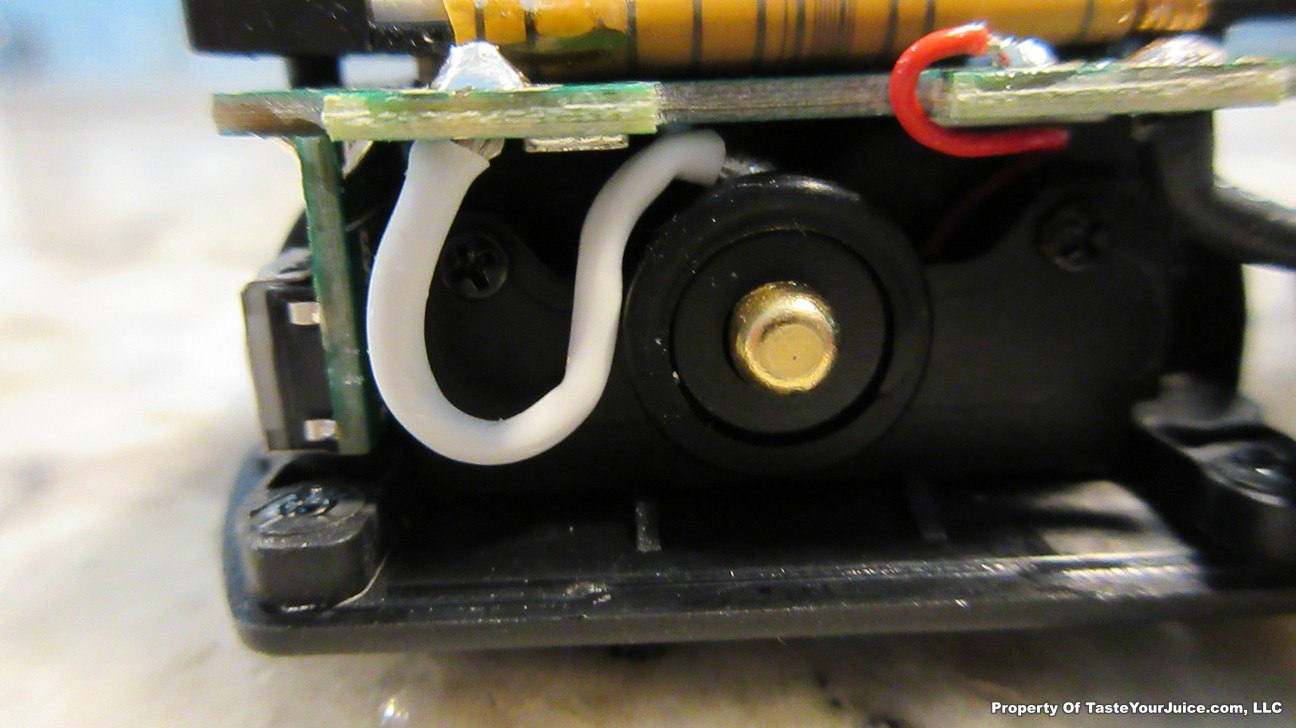
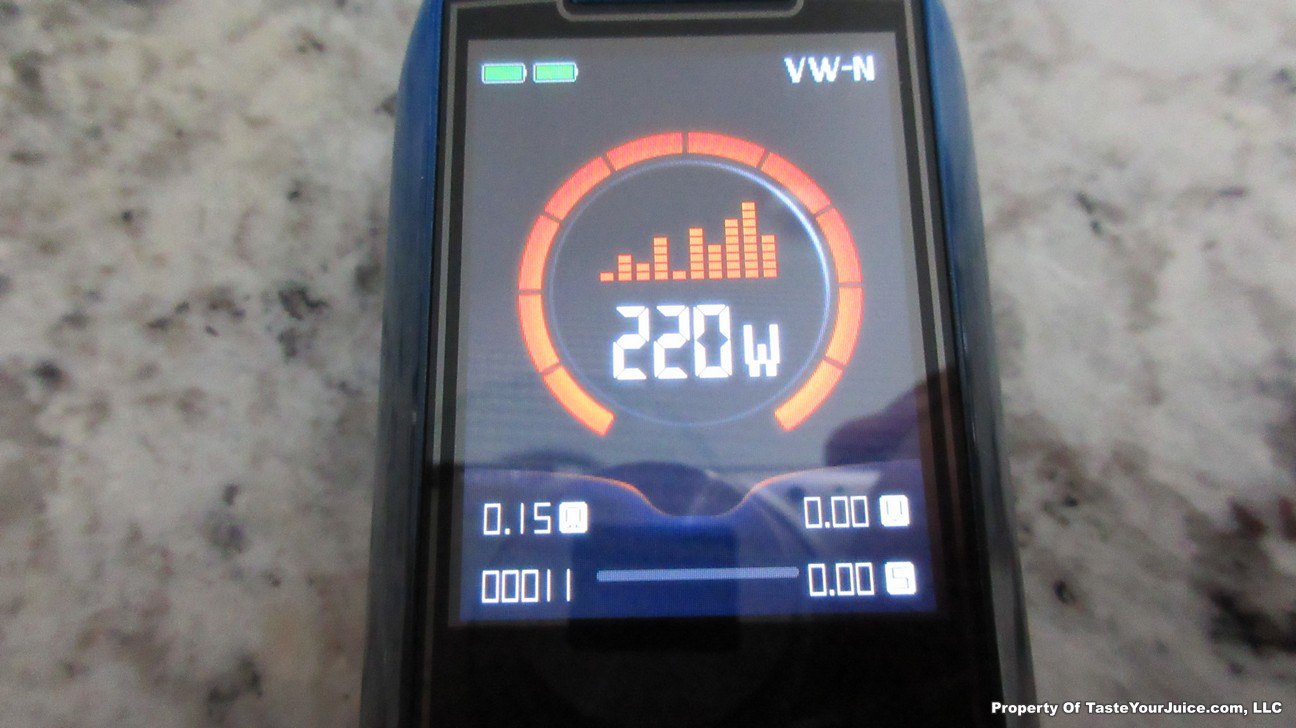
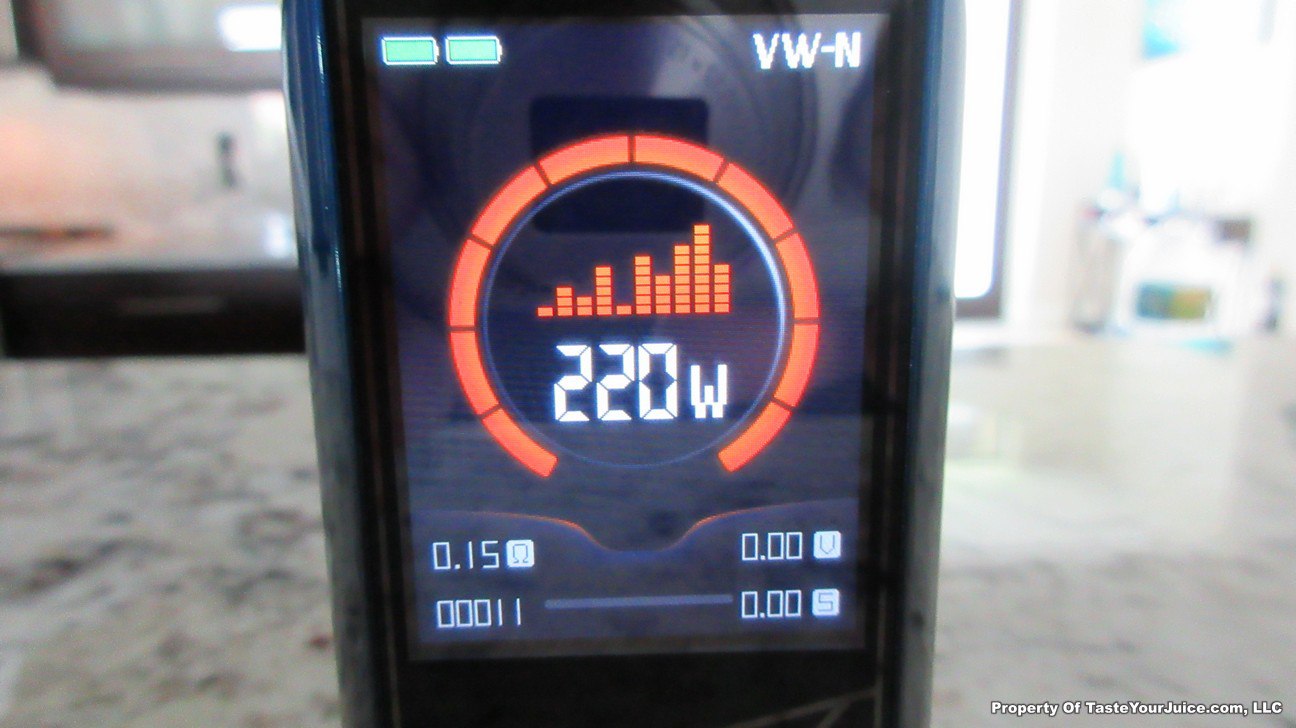
The Photos:
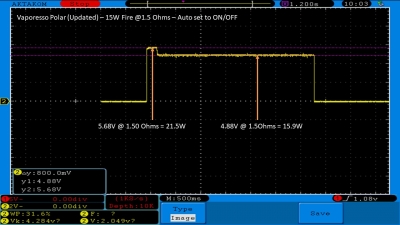
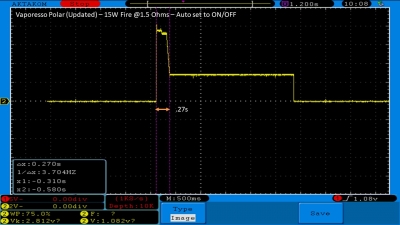
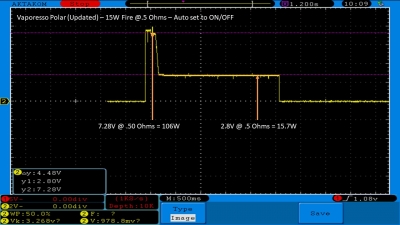
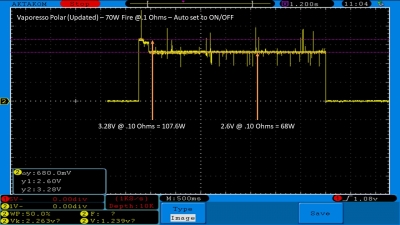
The Test Sheet:
ReviewForm - Vaporesso Polar
NEW FROM REGULATOR WATCH – Deep Vape | Battle to Legalize Vaping in Canada w. Shaun Casey | CVE 2018
As war stories go, no one tells it better than the man at the center of the battle to legalize vaping in Canada: Shaun Casey. As the president of the Canadian Vaping Association, he’s played a pivotal role in guiding the industry through the minefield of what is now newly minted regulation. While at the same time, fighting fiercely for vaper rights and the long-term viability of Canada’s vaping industry.
In this special in-depth edition of RegWatch, we sit down with Casey at Canada’s Vape Expo in Toronto to talk wins, losses and what’s next in the fight to save vaping.
Only on RegWatch by RegulatorWatch.com
Produced by: Brent Stafford
Released: July 11, 2018


































































































































































































































































 Store
Store












